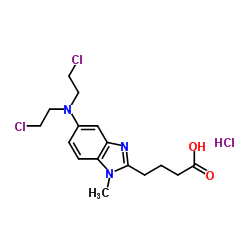
Teva Pharmaceuticals and Eagle Pharmaceuticals Announce FDA Approval of BENDEKA™ (bendamustine hydrochloride) Injection
JERUSALEM & WOODCLIFF LAKE, N.J.--(BUSINESS WIRE)--Dec. 8, 2015-- Teva Pharmaceutical Industries Ltd. (NYSE:TEVA) and Eagle Pharmaceuticals, Inc. (Nasdaq:EGRX) today announce that the U.S. Food and Drug Administration (FDA) has approved BENDEKA™, (bendamustine hydrochloride) injection, a liquid, low-volume (50 mL) and short-time 10-minute infusion formulation of bendamustine. BENDEKA is approved for the treatment of patients with chronic lymphocytic leukemia (CLL) and for the treatment of patients with indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen. Efficacy in CLL relative to first-line therapies other than chlorambucil has not been established.
“We are thrilled that the FDA has approved BENDEKA and are excited for what we believe will be a promising launch with Teva. Importantly, we believe that patients with CLL or indolent B-cell NHL that has progressed will benefit from the multiple administration options this product offers,” said Scott Tarriff, President and Chief Executive Officer of Eagle Pharmaceuticals.
“Teva looks forward to commercializing this new bendamustine product, which we believe represents an important benefit to both patients and healthcare providers,” said Paul Rittman, Senior Vice President and General Manager, Teva Oncology. “We are pleased to add BENDEKA to Teva’s Oncology portfolio, and bendamustine franchise, furthering our commitment to enhancing treatment options for patients affected by cancer.”
BENDEKA was granted Orphan Drug Designations for both CLL and indolent B-cell NHL.
Under the February 2015 exclusive license agreement for BENDEKA, Teva is responsible for all U.S. commercial activities for the product including promotion and distribution. Teva expects to make BENDEKA commercially available to prescribers during the first quarter of 2016.
Indications
BENDEKA is indicated for the treatment of patients with chronic lymphocytic leukemia (CLL). Efficacy relative to first-line therapies other than chlorambucil has not been established.
BENDEKA is indicated for the treatment of patients with indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen.
Source: http://www.tevapharm.com/