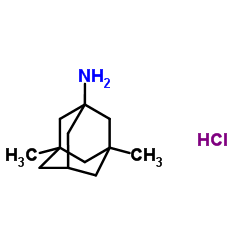
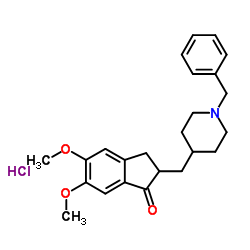
Allergan and Adamas Announce New Expanded Indication for NAMZARIC® (Memantine and Donepezil Hydrochlorides) Extended Release for the Treatment of Moderate to Severe Alzheimer's Disease
memantine hydrochloride donepezil hydrochloride
DUBLIN, Ireland - July 19, 2016 - Allergan plc (NYSE: AGN) and Adamas Pharmaceuticals, Inc. (NASDAQ: ADMS), today announced that the U.S. Food and Drug Administration (FDA) has approved a new, expanded label for NAMZARIC® (memantine and donepezil hydrochlorides) extended-release, a once-daily, fixed-dose combination of memantine hydrochloride (a NMDA receptor antagonist) and donepezil hydrochloride (an acetylcholinesterase inhibitor, AChEI).
With the new indication, patients with moderate to severe Alzheimer's disease, who are currently stabilized on Aricept, donepezil hydrochloride (10 mg), can now start combination therapy directly with NAMZARIC. Approximately 75% of patients diagnosed with Alzheimer's Disease are in the moderate to severe stage of the disease and yet only about one-third of these patients are treated with combination therapy.
"Clinical studies have shown that combination therapy with Namenda XR and an AChEI such as Aricept demonstrated greater improvement in cognition and global function verses an AChEI alone. NAMZARIC offers the benefits of combining two products that each work differently to treat moderate to severe Alzheimer's disease while at the same time reducing the number of pills a patient and their caregivers need to administer each day," says Gavin Corcoran, M.D., chief medical officer at Allergan. "At Allergan, we're proud to continue developing products and supporting new programs that can help patients and their caregivers as they navigate this complex disease."
With the new, expanded indication, NAMZARIC will be available in four dosage strengths which allows patients currently taking Aricept 10mg to start on NAMZARIC the very next day. Namzaric is covered broadly by Medicare Part D prescription plans.
In addition to the two currently available dosage strengths, the two new NAMZARIC dosage strengths will be available in pharmacies in September.
For more information about NAMZARIC, visit www.Namzaric.com.
About the Clinical Trial
The efficacy and safety of the coadministration of memantine HCl extended release and acetylcholinesterase inhibitors (AChEIs), including donepezil HCl, was based on the results of a randomized, double-blind, placebo-controlled trial of 677 patients with moderate to severe Alzheimer's Disease on a stable dose of AChEIs. The clinical study was not conducted with Namazaric; however, bioequivalence of Namzaric with coadministered memantine HCl extended release and donepezil HCl was demonstrated. Approximately 68% of the patients randomized to receive either memantine HCl extended release 28 mg or placebo were taking donepezil as the AchEI at Baseline and throughout the study. The results of this study, demonstrated statistically significant improvement in cognition and global function for patients treated with NAMENDA XR (memantine HCl extended release) 28 mg plus an AChEI compared to placebo plus an AchEI at 24 weeks.
About NAMZARIC®
NAMZARIC is a once-daily, fixed-dose combination of memantine hydrochloride, a NMDA receptor antagonist, and donepezil hydrochloride, an acetylcholinesterase inhibitor indicated for the treatment of moderate to severe dementia of the Alzheimer's type in patients stabilized on 10 mg of donepezil HCl once daily
Memantine hydrochloride extended-release is the active ingredient in the currently marketed NAMENDA XR®, which is indicated for the treatment of moderate to severe dementia of the Alzheimer's type. Donepezil is the active ingredient in ARICEPT® (donepezil hydrochloride), which is indicated for the treatment of mild to severe dementia of the Alzheimer's type. Allergan and Adamas collaborated on the development of the fixed-dose combination and Allergan owns the exclusive U.S. commercialization rights, while Adamas will retain exclusive commercialization rights outside of the U.S.
About Allergan
Allergan plc (NYSE: AGN), headquartered in Dublin, Ireland, is a unique, global pharmaceutical company and a leader in a new industry model – Growth Pharma. Allergan is focused on developing, manufacturing and commercializing innovative branded pharmaceuticals, high-quality generic and over-the-counter medicines and biologic products for patients around the world.Allergan markets a portfolio of best-in-class products that provide valuable treatments for the central nervous system, eye care, medical aesthetics, gastroenterology, women's health, urology, cardiovascular and anti-infective therapeutic categories, and operates the world's third-largest global generics business, providing patients around the globe with increased access to affordable, high-quality medicines. Allergan is an industry leader in research and development, with one of the broadest development pipelines in the pharmaceutical industry and a leading position in the submission of generic product applications globally.With commercial operations in approximately 100 countries, Allergan is committed to working with physicians, healthcare providers and patients to deliver innovative and meaningful treatments that help people around the world live longer, healthier lives.For more information, visit Allergan's website at www.allergan.com.About AdamasAdamas Pharmaceuticals, Inc. (NASDAQ: ADMS) is driven to improve the lives of those affected by chronic disorders of the central nervous system. The company seeks to achieve this by modifying the pharmacokinetic profiles of approved drugs to create novel therapeutics for use alone and in fixed-dose combination products. Adamas is currently developing ADS-5102, its lead wholly-owned product candidate, for the treatment of levodopa-induced dyskinesia associated with Parkinson's disease and for the treatment of major symptoms associated with multiple sclerosis in patients with walking impairment. The company is also evaluating ADS-4101, an extended-release version of an FDA-approved single-agent compound for the treatment of epilepsy. In addition, under a license agreement with Forest Laboratories Holdings Limited, an indirect wholly-owned subsidiary of Allergan plc., the company is eligible to receive royalties from Forest on sales of Namenda XR® and Namzaric™ beginning in June of 2018 and May of 2020, respectively.SOURCE: Allerganhttp://pipelinereview.com/