CAMBRIDGE, MA, USA I August 15, 2016 I
AVEO Oncology (NASDAQ:AVEO) today announced the initiation of a clinical evaluation of AVEO’s oral, once-daily,
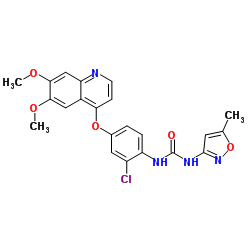
vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI), tivozanib, in combination with Bristol-Myers Squibb’s anti-PD-1 therapy, Opdivo® (nivolumab), in advanced
renal cell carcinoma (RCC). Bristol-Myers Squibb will supply nivolumab for use in the Phase 1/2 AVEO-sponsored TiNivo trial. The trial will be led by the Institut Gustave Roussy in Paris under the direction of Professor Bernard Escudier, MD, Chairman of the Genitourinary Oncology Committee.
The Phase 1 trial will evaluate tivozanib in combination with nivolumab at escalating doses of tivozanib in patients with advanced RCC, and will be followed by an expansion Phase 2 cohort at the established combination dose.
“The introduction of immunotherapies has greatly improved outcomes in renal cell carcinoma, and combination therapy is the obvious next step in advancing treatment,” said Professor Escudier. “There is already early evidence that combining VEGF inhibitors with immune checkpoint inhibitors can improve outcomes, but tolerability of the combination and, in particular, avoiding overlapping liver toxicities, fatigue and stomach disorders is critical to ensuring that both therapies can be delivered at effective levels. Tivozanib’s distinct tolerability profile among VEGF TKIs makes it a potentially unique candidate for use with nivolumab. I look forward to enrolling this study and to understanding how this combination translates to the clinic.”
“Research suggests that, because VEGF inhibition may limit the presence of immunosuppressive cells, VEGF therapy may be an ideal primer and a natural combination for improving the efficacy of anti-PD-1 therapies such as nivolumab, which are designed to reveal the tumor to the immune system,” said Michael Needle, MD, chief medical officer of AVEO. “This study has the potential to unlock a more effective, better tolerated new treatment approach in RCC. We appreciate Bristol-Myers Squibb’s support for this study, and we look forward to working with Dr. Escudier and his team to fully understand this potential.”
About TivozanibTivozanib is an oral, once-daily, vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI). It is a potent, selective and long half-life inhibitor of all three VEGF receptors and is designed to optimize VEGF blockade while minimizing off-target toxicities, potentially resulting in improved efficacy and minimal dose modifications. Tivozanib has been investigated in several tumors types, including renal cell, colorectal and breast cancers.
About AVEOAVEO Oncology (AVEO) is a biopharmaceutical company dedicated to advancing a broad portfolio of targeted therapeutics for oncology and other areas of unmet medical need. The company is focused on developing and commercializing its lead candidate tivozanib, a potent, selective, long half-life inhibitor of vascular endothelial growth factor 1, 2 and 3 receptors, in North America as a treatment for Renal Cell Carcinoma and other cancers. AVEO is leveraging multiple partnerships to develop and commercialize tivozanib in non-oncologic indications worldwide and oncology indications outside of North America, as well as to progress its pipeline of novel therapeutic candidates in cancer and cachexia (wasting syndrome). For more information, please visit the company’s website at www.aveooncology.com.
SOURCE: AVEO Pharmaceuticals
http://pipelinereview.com/
 CAMBRIDGE, MA, USA I August 15, 2016 I AVEO Oncology (NASDAQ:AVEO) today announced the initiation of a clinical evaluation of AVEO’s oral, once-daily, vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI), tivozanib, in combination with Bristol-Myers Squibb’s anti-PD-1 therapy, Opdivo® (nivolumab), in advanced renal cell carcinoma (RCC). Bristol-Myers Squibb will supply nivolumab for use in the Phase 1/2 AVEO-sponsored TiNivo trial. The trial will be led by the Institut Gustave Roussy in Paris under the direction of Professor Bernard Escudier, MD, Chairman of the Genitourinary Oncology Committee.The Phase 1 trial will evaluate tivozanib in combination with nivolumab at escalating doses of tivozanib in patients with advanced RCC, and will be followed by an expansion Phase 2 cohort at the established combination dose.“The introduction of immunotherapies has greatly improved outcomes in renal cell carcinoma, and combination therapy is the obvious next step in advancing treatment,” said Professor Escudier. “There is already early evidence that combining VEGF inhibitors with immune checkpoint inhibitors can improve outcomes, but tolerability of the combination and, in particular, avoiding overlapping liver toxicities, fatigue and stomach disorders is critical to ensuring that both therapies can be delivered at effective levels. Tivozanib’s distinct tolerability profile among VEGF TKIs makes it a potentially unique candidate for use with nivolumab. I look forward to enrolling this study and to understanding how this combination translates to the clinic.”“Research suggests that, because VEGF inhibition may limit the presence of immunosuppressive cells, VEGF therapy may be an ideal primer and a natural combination for improving the efficacy of anti-PD-1 therapies such as nivolumab, which are designed to reveal the tumor to the immune system,” said Michael Needle, MD, chief medical officer of AVEO. “This study has the potential to unlock a more effective, better tolerated new treatment approach in RCC. We appreciate Bristol-Myers Squibb’s support for this study, and we look forward to working with Dr. Escudier and his team to fully understand this potential.”About TivozanibTivozanib is an oral, once-daily, vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI). It is a potent, selective and long half-life inhibitor of all three VEGF receptors and is designed to optimize VEGF blockade while minimizing off-target toxicities, potentially resulting in improved efficacy and minimal dose modifications. Tivozanib has been investigated in several tumors types, including renal cell, colorectal and breast cancers.About AVEOAVEO Oncology (AVEO) is a biopharmaceutical company dedicated to advancing a broad portfolio of targeted therapeutics for oncology and other areas of unmet medical need. The company is focused on developing and commercializing its lead candidate tivozanib, a potent, selective, long half-life inhibitor of vascular endothelial growth factor 1, 2 and 3 receptors, in North America as a treatment for Renal Cell Carcinoma and other cancers. AVEO is leveraging multiple partnerships to develop and commercialize tivozanib in non-oncologic indications worldwide and oncology indications outside of North America, as well as to progress its pipeline of novel therapeutic candidates in cancer and cachexia (wasting syndrome). For more information, please visit the company’s website at www.aveooncology.com.SOURCE: AVEO Pharmaceuticalshttp://pipelinereview.com/
CAMBRIDGE, MA, USA I August 15, 2016 I AVEO Oncology (NASDAQ:AVEO) today announced the initiation of a clinical evaluation of AVEO’s oral, once-daily, vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI), tivozanib, in combination with Bristol-Myers Squibb’s anti-PD-1 therapy, Opdivo® (nivolumab), in advanced renal cell carcinoma (RCC). Bristol-Myers Squibb will supply nivolumab for use in the Phase 1/2 AVEO-sponsored TiNivo trial. The trial will be led by the Institut Gustave Roussy in Paris under the direction of Professor Bernard Escudier, MD, Chairman of the Genitourinary Oncology Committee.The Phase 1 trial will evaluate tivozanib in combination with nivolumab at escalating doses of tivozanib in patients with advanced RCC, and will be followed by an expansion Phase 2 cohort at the established combination dose.“The introduction of immunotherapies has greatly improved outcomes in renal cell carcinoma, and combination therapy is the obvious next step in advancing treatment,” said Professor Escudier. “There is already early evidence that combining VEGF inhibitors with immune checkpoint inhibitors can improve outcomes, but tolerability of the combination and, in particular, avoiding overlapping liver toxicities, fatigue and stomach disorders is critical to ensuring that both therapies can be delivered at effective levels. Tivozanib’s distinct tolerability profile among VEGF TKIs makes it a potentially unique candidate for use with nivolumab. I look forward to enrolling this study and to understanding how this combination translates to the clinic.”“Research suggests that, because VEGF inhibition may limit the presence of immunosuppressive cells, VEGF therapy may be an ideal primer and a natural combination for improving the efficacy of anti-PD-1 therapies such as nivolumab, which are designed to reveal the tumor to the immune system,” said Michael Needle, MD, chief medical officer of AVEO. “This study has the potential to unlock a more effective, better tolerated new treatment approach in RCC. We appreciate Bristol-Myers Squibb’s support for this study, and we look forward to working with Dr. Escudier and his team to fully understand this potential.”About TivozanibTivozanib is an oral, once-daily, vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI). It is a potent, selective and long half-life inhibitor of all three VEGF receptors and is designed to optimize VEGF blockade while minimizing off-target toxicities, potentially resulting in improved efficacy and minimal dose modifications. Tivozanib has been investigated in several tumors types, including renal cell, colorectal and breast cancers.About AVEOAVEO Oncology (AVEO) is a biopharmaceutical company dedicated to advancing a broad portfolio of targeted therapeutics for oncology and other areas of unmet medical need. The company is focused on developing and commercializing its lead candidate tivozanib, a potent, selective, long half-life inhibitor of vascular endothelial growth factor 1, 2 and 3 receptors, in North America as a treatment for Renal Cell Carcinoma and other cancers. AVEO is leveraging multiple partnerships to develop and commercialize tivozanib in non-oncologic indications worldwide and oncology indications outside of North America, as well as to progress its pipeline of novel therapeutic candidates in cancer and cachexia (wasting syndrome). For more information, please visit the company’s website at www.aveooncology.com.SOURCE: AVEO Pharmaceuticalshttp://pipelinereview.com/ 


