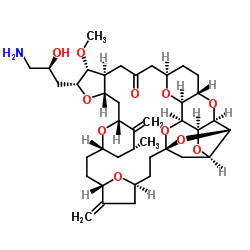
The
US Food and Drug Administration (FDA) has approved the chemotherapy drug
Halaven (eribulin) to treat liposarcoma, a type of soft tissue sarcoma. Halaven is already approved to treat advanced breast cancer. The new use is for people with liposarcoma who have already been treated with anthracycline-based chemotherapy and have tumors that cannot be removed through surgery or have spread to other parts of the body. This is the first drug approved for people with liposarcoma that has shown improvement in overall survival.
Liposarcomas are cancerous tumors of fat tissue. They can develop anywhere in the body, but they most often develop in the thigh, behind the knee, and inside the back of the abdomen.
The FDA based its approval on a clinical trial of 143 people with liposarcoma that couldn’t be removed through surgery or had spread either to nearby lymph nodes or other parts of the body, and had already been treated with chemotherapy. People in the study were given either Halaven or dacarbazine, another chemotherapy drug. Those who received Halaven survived an average 15.6 months compared to 8.4 months for those who received dacarbazine.
Halaven was given priority review, one of the ways in which the FDA tries to speed up availability of drugs to treat serious diseases. Under priority review, the FDA tries to make a decision within 6 months instead of the usual 10 months. Halaven was also granted orphan drug designation, which provides financial incentives to encourage the development of drugs for rare diseases.
The most common side effects of Halaven are fatigue, nausea, hair loss, constipation, nerve damage affecting the hands and feet (peripheral neuropathy), abdominal pain, and fever. Halaven may also cause low levels of infection-fighting white blood cells (neutropenia) or decreased levels of potassium or calcium.
Less common, but more serious side effects include severely decreased levels of infection-fighting white blood cells, severe nerve damage affecting the hands and feet, and changes in heartbeat. It also may cause harm to a developing fetus.
Halaven is marketed by
Eisai.Article date: January 29, 2016
By Stacy Simon
Source: http://www.cancer.org/
 The US Food and Drug Administration (FDA) has approved the chemotherapy drug Halaven (eribulin) to treat liposarcoma, a type of soft tissue sarcoma. Halaven is already approved to treat advanced breast cancer. The new use is for people with liposarcoma who have already been treated with anthracycline-based chemotherapy and have tumors that cannot be removed through surgery or have spread to other parts of the body. This is the first drug approved for people with liposarcoma that has shown improvement in overall survival.Liposarcomas are cancerous tumors of fat tissue. They can develop anywhere in the body, but they most often develop in the thigh, behind the knee, and inside the back of the abdomen.The FDA based its approval on a clinical trial of 143 people with liposarcoma that couldn’t be removed through surgery or had spread either to nearby lymph nodes or other parts of the body, and had already been treated with chemotherapy. People in the study were given either Halaven or dacarbazine, another chemotherapy drug. Those who received Halaven survived an average 15.6 months compared to 8.4 months for those who received dacarbazine.Halaven was given priority review, one of the ways in which the FDA tries to speed up availability of drugs to treat serious diseases. Under priority review, the FDA tries to make a decision within 6 months instead of the usual 10 months. Halaven was also granted orphan drug designation, which provides financial incentives to encourage the development of drugs for rare diseases.The most common side effects of Halaven are fatigue, nausea, hair loss, constipation, nerve damage affecting the hands and feet (peripheral neuropathy), abdominal pain, and fever. Halaven may also cause low levels of infection-fighting white blood cells (neutropenia) or decreased levels of potassium or calcium.Less common, but more serious side effects include severely decreased levels of infection-fighting white blood cells, severe nerve damage affecting the hands and feet, and changes in heartbeat. It also may cause harm to a developing fetus.Halaven is marketed by Eisai.Article date: January 29, 2016By Stacy SimonSource: http://www.cancer.org/
The US Food and Drug Administration (FDA) has approved the chemotherapy drug Halaven (eribulin) to treat liposarcoma, a type of soft tissue sarcoma. Halaven is already approved to treat advanced breast cancer. The new use is for people with liposarcoma who have already been treated with anthracycline-based chemotherapy and have tumors that cannot be removed through surgery or have spread to other parts of the body. This is the first drug approved for people with liposarcoma that has shown improvement in overall survival.Liposarcomas are cancerous tumors of fat tissue. They can develop anywhere in the body, but they most often develop in the thigh, behind the knee, and inside the back of the abdomen.The FDA based its approval on a clinical trial of 143 people with liposarcoma that couldn’t be removed through surgery or had spread either to nearby lymph nodes or other parts of the body, and had already been treated with chemotherapy. People in the study were given either Halaven or dacarbazine, another chemotherapy drug. Those who received Halaven survived an average 15.6 months compared to 8.4 months for those who received dacarbazine.Halaven was given priority review, one of the ways in which the FDA tries to speed up availability of drugs to treat serious diseases. Under priority review, the FDA tries to make a decision within 6 months instead of the usual 10 months. Halaven was also granted orphan drug designation, which provides financial incentives to encourage the development of drugs for rare diseases.The most common side effects of Halaven are fatigue, nausea, hair loss, constipation, nerve damage affecting the hands and feet (peripheral neuropathy), abdominal pain, and fever. Halaven may also cause low levels of infection-fighting white blood cells (neutropenia) or decreased levels of potassium or calcium.Less common, but more serious side effects include severely decreased levels of infection-fighting white blood cells, severe nerve damage affecting the hands and feet, and changes in heartbeat. It also may cause harm to a developing fetus.Halaven is marketed by Eisai.Article date: January 29, 2016By Stacy SimonSource: http://www.cancer.org/ 


