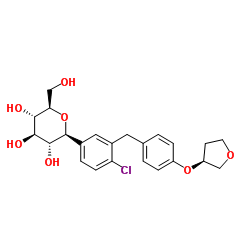
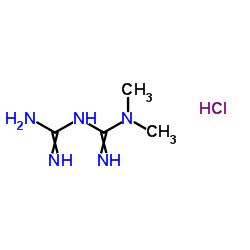
U.S. FDA expands indication for type 2 diabetes treatment Synjardy® (empagliflozin/metformin hydrochloride) to include treatment-naïve adults
empagliflozin metformin hydrochloride
RIDGEFIELD, CT, and INDIANAPOLIS, IN, USA - July 19, 2016 - The U.S. Food and Drug Administration has approved an expanded indication for Synjardy® (empagliflozin and metformin hydrochloride) tablets to include treatment-naïve adults with type 2 diabetes (T2D). SYNJARDY, from Boehringer Ingelheim and Eli Lilly and Company (NYSE: LLY), is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2D when treatment with both empagliflozin and metformin is appropriate.
SYNJARDY is a combination of empagliflozin (Jardiance®) and metformin — two medicines with complementary mechanisms of action — to help control blood glucose in adults with T2D. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, removes excess glucose through the urine by blocking glucose re-absorption in the kidney. Metformin, a commonly prescribed initial treatment for T2D, lowers glucose production by the liver and its absorption in the intestine."Type 2 diabetes is a complex condition, which often requires that people take more than one treatment to manage their blood sugar," said Paul Fonteyne, president and CEO, Boehringer Ingelheim Pharmaceuticals, Inc. "The expanded indication for SYNJARDY further validates the potential of this combination therapy to help adults with type 2 diabetes who are not at goal, including those already being treated and, now, those at the beginning of their treatment journey."The SYNJARDY label was updated to include results from a phase III, double-blind, randomized, active-controlled study that evaluated the efficacy and safety of empagliflozin in combination with metformin as initial therapy compared with the individual components. In the study, at 24 weeks, the combination of empagliflozin 10 mg or 25 mg with metformin 1000 mg or 2000 mg resulted in significant reductions in A1C (a measure of average blood glucose over the past two to three months) compared with the corresponding dose of either component alone.SYNJARDY can cause serious side effects, including Lactic Acidosis (a buildup of lactic acid in the blood). Metformin, one of the medicines in SYNJARDY, can cause lactic acidosis, a rare, but serious condition that can cause death. Lactic acidosis is a medical emergency and must be treated in a hospital. SYNJARDY is not for the treatment of type 1 diabetes or diabetic ketoacidosis.About DiabetesApproximately 29 million Americans and an estimated 415 million people worldwide have diabetes, and nearly 28 percent of Americans with diabetes — totaling 8 million people — are undiagnosed. In the U.S., approximately 12 percent of those aged 20 and older have diabetes. T2D is the most common type, accounting for an estimated 90 to 95 percent of all diagnosed adult diabetes cases in the U.S. Diabetes is a chronic condition that occurs when the body either does not properly produce, or use, the hormone insulin.
What is SYNJARDY?SYNJARDY is a prescription medicine that contains 2 diabetes medicines, empagliflozin (JARDIANCE) and metformin. SYNJARDY can be used along with diet and exercise to lower blood sugar in adults with type 2 diabetes.
SYNJARDY is not for people with type 1 diabetes, or for people with diabetic ketoacidosis (increased ketones in the blood or urine).SOURCE: Eli Lilly
Published on Tuesday, 19 July 2016
http://pipelinereview.com/