US FDA refuses to approve Merck's Zetia and Vytorin medicines
The US Food and Drug Administration (FDA) has refused to approve Zetia and Vytorin, Merck's medicines that are used to reduce the risk of cardiovascular events in patients with coronary heart disease.
The FDA's decision has been announced by Merck, which received a complete response letter from the agency concerning the company's supplemental new drug applications for Zetia and Vytorin.
Merck said that the applications were based on the results of Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT).
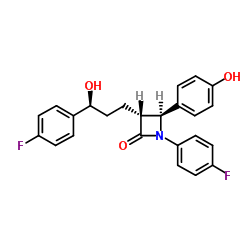
Zetia and Vytorin are indicated for use along with a healthy diet to reduce elevated low-density lipoprotein (LDL) cholesterol in patients with hyperlipidemia.
The effect of Zetia on cardiovascular morbidity and mortality has not been determined.
Zetia, consumed alone or in combination with a statin, is indicated as adjunctive therapy to diet for the reduction of elevated total cholesterol, LDL cholesterol, apolipoprotein B, and non-HDL cholesterol in patients with primary hyperlipidemia when diet alone is not enough.
People with hypersensitivity to any component of the medication should not consume Zetia.
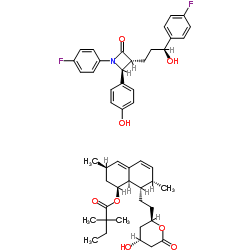
Vytorin, which contains ezetimibe and simvastatin, has shown no incremental benefit on cardiovascular morbidity, and mortality over and above that demonstrated for simvastatin has been established.
It should not be consumed along with strong CYP3A4 inhibitors; or with gemfibrozil, cyclosporine, or danazol.
In addition to reducing elevated total cholesterol, LDL cholesterol, apolipoprotein B, triglycerides, and non-HDL cholesterol, Vytorin is indicated to increase HDL cholesterol in patients with primary hyperlipidemia or mixed hyperlipidemia when diet alone is not enough.
VYTORIN should not be taken with strong CYP3A4 inhibitors; or with gemfibrozil, cyclosporine, or danazol.
In December last year, a FDA advisory committee voted against Merck's claims that Vytorin reduces the risk of heart attacks and strokes in patients with coronary heart disease, Reuters reported.
16 February 2016
Source: http://www.pharmaceutical-technology.com/