Alexion to Acquire Wilson Therapeutics
NEW HAVEN, Conn.--(BUSINESS WIRE)--Alexion Pharmaceuticals, Inc. (NASDAQ:ALXN) and Wilson Therapeutics AB (publ) announced today that Alexion has made a recommended public cash offer to the shareholders in Wilson Therapeutics to acquire all outstanding shares in Wilson Therapeutics by way of a tender offer, through a wholly-owned subsidiary. Wilson Therapeutics is a biopharmaceutical company, based in Stockholm, Sweden, that develops novel therapies for patients with rare copper-mediated disorders. Wilson Therapeutics’ product, WTX101, is in Phase 3 development as a treatment for Wilson disease, a rare genetic disorder with devastating hepatic and neurological consequences for patients. WTX101 is a first-in-class oral copper-binding agent with a unique mechanism of action and ability to access and bind copper from serum and promote its removal from the liver. WTX101 has received Fast Track designation in the U.S. and Orphan Drug Designation for the treatment of Wilson disease in the U.S. and EU.
“Wilson disease is a rare disorder that can lead to severe liver disease, including cirrhosis and acute liver failure, as well as debilitating neurological morbidities such as impaired movement, gait, speech, swallowing, and psychiatric disorders. WTX101 is an innovative product that addresses the underlying cause of the disease and has the potential to define a new standard of care in treating Wilson disease, an area that has not had a new treatment in over two decades,” said Ludwig Hantson, Chief Executive Officer of Alexion. “The acquisition of Wilson Therapeutics is a strong strategic fit for Alexion given the overlap with our current clinical and commercial focus on metabolic and neurologic disorders, and is an important first step in rebuilding our clinical pipeline.”
“Alexion is a global leader in rare diseases with a proven record of developing and commercializing therapies for patients with rare diseases, making them a great partner to make WTX101 available to Wilson disease patients worldwide,” said Jonas Hansson, CEO of Wilson Therapeutics.
The Transaction
Alexion will acquire Wilson Therapeutics through a tender offer that was launched this morning at 7:00 a.m. CET/1:00 a.m. EDT whereby Alexion, through a wholly owned subsidiary, has offered SEK 232 in cash for each outstanding share of Wilson Therapeutics. The total equity value of the transaction amounts to SEK 7,100 million, based on outstanding shares on a fully diluted basis, or approximately $855 million. The Independent Committee of the Board of Directors of Wilson Therapeutics has unanimously recommended Wilson Therapeutics shareholders accept the offer and Alexion’s Board of Directors also unanimously approved the offer. Alexion has obtained shareholder support agreements from the four largest shareholders accounting for 57.4% of Wilson Therapeutics’ outstanding shares and two additional shareholders accounting for 8.7% for a total of 66.1% of Wilson Therapeutics’ outstanding shares, to the effect that these shareholders have undertaken to accept the offer on certain terms. In addition, Polar Capital, holding 7.3% of Wilson Therapeutics’ outstanding shares, has expressed its support for, and intends to accept, the Offer, for a total support of 73.4%. The acquisition of Wilson Therapeutics requires approval of relevant regulatory authorities, and Alexion expects to obtain such approvals prior to the end of the acceptance period. The tender offer is expected to complete and the transaction is expected to close in the second quarter of 2018. Alexion intends to finance the acquisition through cash on hand.
BofA Merrill Lynch is acting as Alexion’s lead financial advisor. Deutsche Bank is also serving as a financial advisor, and DNB Markets is acting as Nordic financial advisor and Settlement Agent. Advokatfirman Cederquist is acting as Alexion’s legal advisor as to Swedish law, and Ropes & Gray LLP is acting as legal advisor as to U.S. law in connection with the Offer. Lazard is acting as exclusive financial adviser to Wilson Therapeutics, and Vinge is serving as legal counsel to Wilson Therapeutics.
About Wilson Disease
Wilson disease is a rare, chronic, genetic and potentially life-threatening liver disorder of impaired copper transport. Copper balance is normally maintained in the body by hepatic excretion of excessive copper in the bile. In patients with Wilson disease, a genetic mutation disables this biliary excretion pathway and excess copper accumulates over time in the liver cells. The accumulation of copper eventually overwhelms safe storage capacity and cellular injury occurs. When the liver’s capacity for copper storage is exceeded, and when liver cells are injured, copper is released into the circulation and may accumulate in other organs, including the central nervous system. Untreated, Wilson disease leads to various combinations and severity of hepatic, neurologic and psychiatric symptoms, and can be fatal.1,2
Wilson disease affects approximately one in every 30,000 people worldwide.3 The average age of diagnosis is 15-20 years,3 with the majority of patients presenting between the ages of 10 and 30.4 Current standard of care includes metal chelators to remove serum copper, followed by maintenance with zinc to prevent re-accumulation.1,2 There have been no new treatment options approved in over two decades and a significant unmet need still exists for patients.
About WTX101
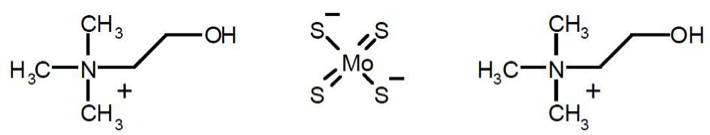
WTX101 (bis-choline tetrathiomolybdate) is a first-in-class copper-binding agent with a unique mechanism of action, under investigation as a novel therapy for Wilson disease. In contrast to current treatments, WTX101 provides an alternative copper-protein binding mechanism by forming a tripartite complex with copper and albumin. WTX101 thereby detoxifies excess copper in both the liver and blood, and promotes copper clearance through biliary excretion (the body’s natural route of elimination). WTX101 has a 10,000-fold higher affinity for copper than other chelators and addresses the underlying cause of the disease.
A Phase 2 study evaluating the efficacy and safety of WTX101 in patients with Wilson disease has been completed successfully.5 In addition, the active moiety of WTX101, tetrathiomolybdate, has been tested in several previous clinical studies in Wilson disease patients. The data from these studies suggest that WTX101 can reduce and control free copper levels and improve symptoms and associated disabilities. The data also suggest that WTX101 is generally well tolerated with a low risk of drug-induced neurological worsening. WTX101 has received Fast Track designation in the U.S. and Orphan Drug Designation for the treatment of Wilson disease in the U.S. and EU.
About Wilson Therapeutics
Wilson Therapeutics is a biopharmaceutical company, based in Stockholm, Sweden, that develops novel therapies for patients with rare copper-mediated disorders. Wilson Therapeutics’ product, WTX101, is in Phase 3 development as a novel treatment for Wilson disease. Wilson Therapeutics is listed in the Mid Cap segment on Nasdaq Stockholm with the stock ticker WTX.
About Alexion
Alexion is a global biopharmaceutical company focused on serving patients and families affected by rare diseases through the innovation, development, and commercialization of life-changing therapies. Alexion is the global leader in complement inhibition and has developed and commercializes the first and only approved complement inhibitor to treat patients with paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), and anti-acetylcholine receptor (AchR) antibody-positive generalized myasthenia gravis (gMG). In addition, Alexion has two highly innovative enzyme replacement therapies for patients with life-threatening and ultra-rare metabolic disorders, hypophosphatasia (HPP) and lysosomal acid lipase deficiency (LAL-D). As the leader in complement biology for over 20 years, Alexion focuses its research efforts on novel molecules and targets in the complement cascade, and its development efforts on the core therapeutic areas of hematology, nephrology, neurology, and metabolic disorders.
April 11, 2018
http://news.alexion.com/


