Arena Pharmaceuticals Reports Positive Phase 2 Results from the OASIS Trial for Etrasimod in Patients with Ulcerative Colitis
SAN DIEGO, March 19, 2018 /PRNewswire/ -- Arena Pharmaceuticals, Inc. (NASDAQ: ARNA), today announced positive topline Phase 2 results from the OASIS trial for etrasimod, an investigational, once-daily, orally administered, selective sphingosine 1-phosphate (S1P) receptor modulator in development for the treatment of ulcerative colitis (UC). Patients receiving the 2 mg dose of etrasimod achieved statistically significant improvements versus placebo in the primary, all secondary, and clinical remission endpoints.
Relative to placebo, there was a statistically significant (p = 0.009) 0.99 point improvement in a 3-component (stool frequency, rectal bleeding and findings on endoscopy) Mayo Clinic Score (ranging from 0 to 9) with etrasimod 2 mg at week 12. In the 1 mg group, there was a 0.43 point improvement in 3-component Mayo Clinic Score at week 12 relative to placebo, which was not statistically significant (p = 0.146). Significantly more patients in the etrasimod 2 mg group achieved endoscopic improvement compared with placebo (41.8% vs. 17.8%, p = 0.003).
The proportion of patients achieving clinical remission, defined by the 3-component Mayo Clinic Score, was 33.0% in the etrasimod 2 mg group compared to 8.1% for the placebo group (p < 0.001). Remission as defined by the 4-component Total Mayo Clinic Score was 24.5% and 6.0% for etrasimod 2 mg and placebo, respectively (p = 0.004).
Etrasimod was well tolerated and there were fewer patients with serious adverse events (SAEs) compared to placebo (0% in 2 mg, 5.8% in 1 mg and 11.1% in placebo). Impact on heart rate and atrioventricular (AV) conduction was low throughout the study with no discontinuations from study related to bradycardia or AV block. There were no increases in liver function tests compared to placebo and no reports of macular edema or pulmonary function test abnormalities. The Company plans to present full study results at future medical congresses.
"The results of this Phase 2 trial are impressive and demonstrate statistically significant efficacy of orally administered etrasimod, including clinically meaningful improvement in remission, as well as endoscopic improvement in what has been historically referred to as mucosal healing," said William Sandborn, M.D., Professor of Medicine and Chief, Division of Gastroenterology and Director, University of California San Diego Inflammatory Bowel Disease Center. "Despite recent advances in treatment options, there remains a significant unmet need for new oral therapies for ulcerative colitis. I look forward to etrasimod advancing into a Phase 3 program."
Preston Klassen, M.D., M.H.S., Executive Vice President, Research and Development and Chief Medical Officer of Arena Pharmaceuticals, said, "We believe these data support proceeding to a Phase 3 program in ulcerative colitis and continuing efforts to understand the broad potential utility of etrasimod in other immune and inflammatory diseases with significant unmet needs. Along with the positive Phase 2 results for ralinepag reported last year, this important milestone for the Company further amplifies our conviction in Arena's internally discovered and developed compounds and their potential to be best-in-class."
About OASIS
OASIS was a randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study to assess safety and efficacy of two orally administered doses (1 mg and 2 mg) of etrasimod in patients with ulcerative colitis (UC) across 71 sites in 16 countries. The OASIS trial randomized 156 patients, with moderate to severe UC (3-component Mayo Clinic Score of 4-9 with an endoscopic subscore ≥ 2 and a rectal bleeding score ≥ 1). The pre-specified statistical analysis plan applied one-sided testing, in which conventional statistical significance is achieved at p-values < 0.025.
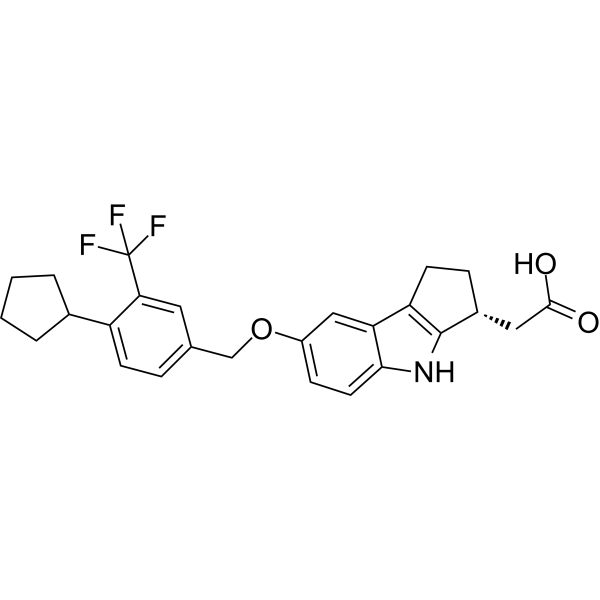
About Etrasimod
Etrasimod (APD334), is an oral, once-daily, next generation, selective sphingosine 1-phosphate (S1P) receptor modulator, discovered by Arena, designed to provide systemic and local cell modulation by targeting S1P receptor subtypes 1, 4 and 5, while avoiding subtypes 2 and 3. Etrasimod is believed to exhibit potentially best-in-class pharmacokinetics and pharmacodynamics with rapid onset of action and rapid recovery of T lymphocytes. Selective binding with S1P receptor subtype 1 is believed to inhibit a specific subset of activated lymphocytes from migrating to sites of inflammation. The result is a reduction of circulating T and B lymphocytes that leads to anti-inflammatory activity while immune surveillance is maintained. The receptor subtypes 4 and 5 exhibit similar activity on additional proliferating immune cell types. Optimized pharmacology and pharmacokinetics may allow improved clinical utility across a broad range of immune and inflammatory conditions.
Etrasimod is an investigational compound not approved for any use in any country.
About Arena Pharmaceuticals
Arena Pharmaceuticals is focused on developing novel, small molecule drugs with optimized receptor pharmacology and pharmacokinetics designed to deliver broad clinical utility across several therapeutic areas. Arena's proprietary pipeline includes potentially first- or best-in-class programs. The most advanced investigational clinical programs are ralinepag (APD811), which is anticipated to commence a Phase 3 program for pulmonary arterial hypertension (PAH), and etrasimod (APD334), for which the Company intends to commence a Phase 3 program for ulcerative colitis (UC) and which the Company believes has potential utility for a broad range of immune and inflammatory conditions. Arena is also evaluating APD371 in Phase 2 for the treatment of pain associated with Crohn's disease. In addition, Arena has collaborations with the following pharmaceutical companies: Everest Medicines Limited (ralinepag and etrasimod in Greater China and select Asian countries), Axovant Sciences GmbH(nelotanserin - Phase 2), Boehringer Ingelheim International GmbH (undisclosed target - preclinical), and Eisai Co., Ltd. and Eisai Inc. (BELVIQ® - marketed product).
http://invest.arenapharm.com/



