GSK's two-drug HIV regimen wins European panel approval
(Reuters) - A panel of European Medicines Agency on Friday recommended approval for GlaxoSmithKline’s two-drug regimen to treat HIV, the virus that causes AIDS.
The Committee for Medicinal Products for Human Use (CHMP) backed the two-drug combination, which is aimed at lessening the side effect of current treatments that combine three or four medicines.
The combination was approved by the U.S. Food and Drug Administration in November. The CHMP opinion sets the stage for a likely approval by the European Commission, which is expected toward the end of the second quarter of 2018, GSK said in a statement.
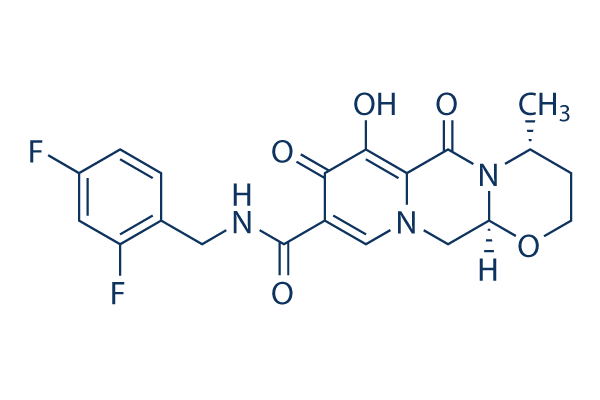
The new HIV treatment, Juluca, is a fixed-dose once-daily tablet that combines two previously approved drugs, dolutegravir and rilpivirine, and is available to patients who have been on a stable regimen for at least six months.
Juluca is produced by GSK’s majority-owned ViiV Healthcare, in which Pfizer and Shionogi also have small stakes.
ViiV’s integrase inhibitor drug dolutegravir is part of GSK’s traditional triple-therapy used to control HIV, while rilpivirine is a Johnson & Johnson drug.
MARCH 23, 2018
https://www.reuters.com/


