Clovis Oncology, Inc. is discontinuing its sponsored Phase 2 open-label monotherapy clinical trial evaluating rucaparib in recurrent, metastatic bladder cancer
The decision is based on recommendations by an independent data monitoring committee (“DMC”) following its review of preliminary efficacy data for 62 patients enrolled and treated in the study, which demonstrated that the objective response rate in the intent-to-treat population does not meet the protocol-defined continuance criteria, and suggests that treatment with monotherapy rucaparib may not provide a meaningful clinical benefit to patients.
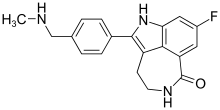
Therefore, the DMC recommended to stop enrollment to the study, and Clovis has decided to terminate the ATLAS trial early. The recommendation of the DMC was not based on the safety profile of rucaparib in this study population.
Clovis is continuing to evaluate the potential for rucaparib in combination with other agents for the treatment of advanced bladder cancer. Clovis also plans to enroll patients with advanced bladder cancer and selected genetic mutations in a planned pan-tumor trial of rucaparib expected to begin in the second half of 2019.
April 12, 2019
https://www.sec.gov/


