Regulators hit Italian drugmaker with warning over manufacturing issues
Italian regulators slapped a warning letter on ICI International Chemical Industry for failing to comply with good manufacturing practices at its Cellole, Italy plant.
The Italian Medicines Agency found in a March 22 inspection of the facility that there were 19 major and nine other deficiencies related to “inadequate pharmaceutical quality systems that resulted in a number of issues.”
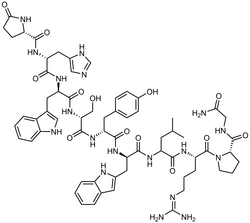
Triptorelin
Those issues included poor deviations management and investigation, lack of documentation handling, lack of cleaning validation, lack of process validation, as well as a lack of analytical review and method validation and poor computer system validation.
The agency called for the suspension of the company’s manufacturing authorization and withdrawal of its current valid GMP certificate.
The IMA cited the drug Spherotide (triptorelin), which is used to treat prostate cancer, in its letter posted on the EudraGDMP website.
According to a Bloomberg company profile, International Chemical was founded in 1989 and manufactures, fabricates or processes pharmaceuticals for human and veterinary use.
May 28, 2019
https://www.fiercepharma.com/


