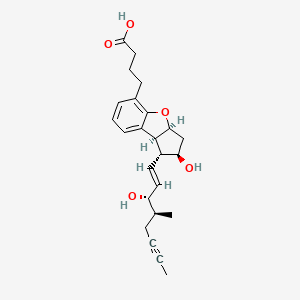
United Theraputics ends esuberaprost trial early
Maryland-based United Theraputics has ended its late-stage trial for esuberaprost tablets in patients suffering from pulmonary arterial hypertension, due to the trial not meeting its primary endpoint.
The 240-patient trial investigated the addition of esuberaprost to the company's inhaled PAH treatment Tyvaso (treprostinil) compared to Tyvaso alone.
Secondary endpoint data was not mentioned, which included goals of six-minute walking distance, Borg dyspnoea score and WHO functional class.
The company kept the announcement short, but shared the information that in the Phase III BEAT clinical study, the drug didn’t achieve delayed time to first clinical worsening event, causing the company to choose to “discontinue further esuberaprost development.”
PAH is one type of a broader disease called pulmonary hypertension, meaning high blood pressure in the lungs. In PAH, the increased pressure is caused by obstruction in the small arteries in the lungs. Symptoms of PAH are similar to those seen in many other common diseases, such as asthma, emphysema or chronic obstructive pulmonary disease (COPD).
On the news, shares in the company dropped 5.7%.
9th April 2019
http://www.pharmatimes.com/