Bayer: Vericiguat is primarily tested by the FDA in the USA
Bayer’s heart failure drug Vericiguat has received the status of “Priority Review” from the US agency FDA and is therefore a priority. This means that the FDA has to decide on the application for approval within six months instead of the usual twelve months – in this case, this is no later than January 21 of the coming year.
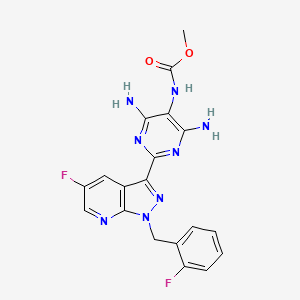
Bayer’s approval application is based on the recently published positive results of the phase III study with Vericiguat, which was developed in cooperation with MSD. This is to be used for therapy in patients with symptomatic chronic heart failure with reduced ejection performance of less than 45 percent after a worsening event.
Heart failure is the leading cause of hospitalization for people over 65 and affects more than 60 million people worldwide. Events of deterioration and the associated frequent hospital stays, unfortunately also the death of the patient, are a sad reality with heart failure even under currently available therapies.
20 Jul 2020
https://personal-financial.com/



