Bristol Myers Squibb to Acquire MyoKardia for $13.1 Billion in Cash
NEW YORK & BRISBANE, Calif.--(BUSINESS WIRE)-- Bristol Myers Squibb (NYSE: BMY) and MyoKardia, Inc. (Nasdaq: MYOK) today announced a definitive merger agreement under which Bristol Myers Squibb will acquire MyoKardia for $13.1 billion, or $225.00 per share in cash. The transaction was unanimously approved by both the Bristol Myers Squibb and MyoKardia Boards of Directors and is anticipated to close during the fourth quarter of 2020.
MyoKardia is a clinical-stage biopharmaceutical company discovering and developing targeted therapies for the treatment of serious cardiovascular diseases. Through the transaction, Bristol Myers Squibb gains mavacamten, a potential first-in-class cardiovascular medicine for the treatment of obstructive hypertrophic cardiomyopathy (“HCM”), a chronic heart disease with high morbidity and patient impact. A New Drug Application (“NDA”) for mavacamten for the treatment of symptomatic obstructive HCM – based on data from the EXPLORER-HCM study – is expected to be submitted to the U.S. Food and Drug Administration (“FDA”) in the first quarter of 2021. Bristol Myers Squibb expects to explore the full potential of mavacamten in additional indications, including non-obstructive HCM, as well as develop MyoKardia’s promising pipeline of novel compounds, including two clinical-stage therapeutics: danicamtiv (formerly MYK-491) and MYK-224.
“The acquisition of MyoKardia further strengthens our portfolio, pipeline and scientific capabilities, and is expected to add a meaningful medium- and long-term growth driver,” said Giovanni Caforio, M.D., Board Chair and Chief Executive Officer of Bristol Myers Squibb. “We are further strengthening our outstanding cardiovascular franchise through the addition of mavacamten, a promising medicine with the potential to address a significant unmet medical need in patients with cardiovascular disease. Our companies share a commitment to innovation and bold science, and our respective strengths will help us realize the value inherent in this portfolio. We have long admired MyoKardia and what they have done to revolutionize cardiovascular treatments through a precision medicine approach. We look forward to welcoming their talented team to our company.”
“MyoKardia was formed eight years ago with the aim of changing the world for people with serious cardiovascular diseases through bold and innovative science. Since then, MyoKardia’s dedicated employees have established an unparalleled pipeline of targeted therapeutics designed to change the course of disease and return the heart to normal function,” said Tassos Gianakakos, Chief Executive Officer of MyoKardia. “Bristol Myers Squibb shares our vision for transforming the treatment of cardiovascular disease. They value our team and the potential of our platform and, most importantly, share our unwavering commitment to placing patients at the center of everything we do. Together, our complementary strengths and expanded resources and reach will further accelerate the pace at which we can discover, develop and commercialize our novel medicines for the benefit of people suffering from cardiovascular disease around the world.”
Mavacamten
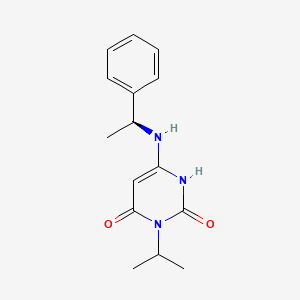
Bristol Myers Squibb expects the transaction, when complete, to:
Further strengthen the company’s outlook with the addition of mavacamten, which has significant commercial potential in the lead indication, obstructive HCM, and upside in additional future indications, including non-obstructive HCM .
With the high unmet medical need in obstructive HCM, mavacamten can be a significant medium- and long-term growth driver. Mavacamten demonstrated clinically meaningful results in the pivotal Phase 3 EXPLORER-HCM trial, meeting the primary and all secondary endpoints, and showed meaningful improvements in symptoms, functional status and quality of life by reducing the obstruction of blood flow from the heart. This potential first-in-class medicine, for which an NDA is expected to be submitted to the FDA in the first quarter of 2021, may help to change the course of the disease.
Accelerate the expansion of Bristol Myers Squibb’s cardiovascular portfolio.
Bristol Myers Squibb has established Eliquis® (apixaban) as the #1 oral anticoagulant globally with a best-in-class profile, driven by leading commercial execution.Mavacamten will be a fully owned asset that fits well into Bristol Myers Squibb’s existing portfolio, given the company’s broad expertise in cardiovascular disease. Through this acquisition, Bristol Myers Squibb gains MyoKardia’s critical talent and capabilities on the U.S. West Coast, which will support fully realizing the opportunity in obstructive HCM and exploring the full potential of mavacamten in additional indications. Bristol Myers Squibb will also be well positioned to advance the global development of MyoKardia’s portfolio of clinical- and early-stage pipeline candidates, while continuing to advance its existing Factor XIa inhibitor program.
Deliver significant financial benefits.
The transaction is expected to add a significant growth driver during the medium- to long-term. It is expected to be minimally dilutive to Bristol Myers Squibb’s non-GAAP earnings per share (EPS) in 2021 and 2022 and accretive beginning in 2023. Bristol Myers Squibb reaffirms its existing 2021 non-GAAP EPS guidance range. There is no reliable estimable comparable GAAP measure as described below.
10/05/2020
https://news.bms.com/



