Chi-Med's surufatinib bags US fast track designations
US regulators have granted two fast track designations for the development of Chi-Med's surufatinib for the treatment of both advanced and progressive pancreatic neuroendocrine tumours (NET) and extra-pancreatic (non-pancreatic) NET.
The drug, a novel, oral angio-immuno kinase inhibitor, is being developed for patients who are unable to have surgery for these conditions.
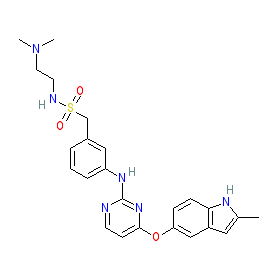
It's dual mechanism of action selectively inhibits the tyrosine kinase activity associated with vascular endothelial growth factor receptor and fibroblast growth factor receptor, which both inhibit angiogenesis, and colony stimulating factor-1 receptor, which regulates tumour-associated macrophages, promoting the body’s immune response against tumour cells.
Fast track designation could help accelerate the drug's development and review, in the hope that it will fulfil unmet medical needs.
17th April 2020
http://www.pharmatimes.com/news/



