FDA OKs Epizyme's tazemetostat for treatment-resistant follicular lymphoma
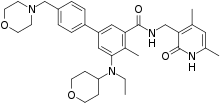
Under accelerated review status, the FDA approves Epizyme's (EPZM +4.5%) Tazverik (tazemetostat) for the treatment of adult patients with relapsed/refractory follicular lymphoma (FL) whose tumors are positive for an EZH2 mutation as detected by an FDA-approved test and who have received at least two prior systemic therapies and adults with relapsed/refractory FL who have no realistic treatment options.
Accelerated approval stipulates certain post-marketing requirements including a confirmatory study to validate the treatment benefits.
The FDA approved the methyltransferase inhibitor in January for epithelioid sarcoma.
Jun. 18, 2020
https://seekingalpha.com/



