FDA spanks KVK Tech over impurities in ADHD drug
The FDA has found issues with the testing practices of a U.S. generics maker that had specific problems with an ADHD drug as well as one for weight loss.
The FDA, in a warning letter posted this week, says, among other problems, KVK Tech of Newtown, Pennsylvania, failed to properly integrate co-eluting peaks during impurity testing of its phentermine HCL capsules, a drug used for weight loss. As a result, the letter says the analysis failed to detect out-of-specification results for at least one lot of the drug.
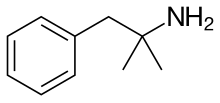
KVK Tech said even though its methods might underestimate impurities in the drug, the impact was minimal because “the failing batches were ultimately recalled from the market, albeit at a potentially later time.”
The FDA, unimpressed by this argument, ordered the company to bring in an independent consultant to help it assess its laboratory practices and then put together a plan on how it intends to bring them up to agency expectations.
The letter also found problems with the plant’s investigation of foreign particles found in a lot of the ADHD drug methylphenidate oral solution. The company said the particles were introduced during filling by wipes used in cleaning equipment. But the FDA said KVK Tech had not adequately investigated the particles and their potential effect on the drug’s quality before releasing the lot.
In addition to providing the agency with a thorough assessment of how it investigates deviations, the FDA asked for an independent assessment of four years worth of manufacturing records “to determine if manufacturing events” that should have been uncovered had been missed.
Feb 26, 2020
https://www.fiercepharma.com/



