Jazz’s Xywav, a low-sodium alternative to blockbuster Xyrem, snags FDA nod in narcolepsy
Jazz Pharmaceuticals’ Xyrem has become the standard of care for two symptoms of narcolepsy since its FDA approval in 2002. But the company’s introducing a sister drug that potentially offers better heart safety.
On Tuesday, the FDA approved Jazz’s Xywav, previously known as JZP-258, for sudden muscle weakness or excessive daytime sleepiness in narcolepsy patients 7 years of age and older.
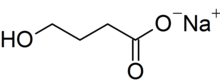
Xywav is a low-sodium version of Xyrem, which carries warnings about its high sodium content. Both drugs contain the active ingredient oxybate, but the new offering has 92% less sodium, or about 1g to 1.5g per night, than Xyrem at the recommended dosage.
Because excess sodium intake is linked to increase risk of heart disease, Jazz argues Xywav could be the safer oxybate treatment option by helping patients better manage their daily sodium intake.
In a 201-patient phase 3 trial, those who got Xywav after a drug-switching and titration phase continued to experience low rates of cataplexy attacks and excessive daytime drowsiness, while patients taking placebo had significant increases in those symptoms.
Xyrem is Jazz’s top-selling med, with 2019 sales growing 17% year over year to reach $1.64 billion. “We believe there is a market growth opportunity for JZP-258 in patients currently not prescribed Xyrem due to sodium and cardiometabolic considerations,” Jazz CEO Bruce Cozadd said during a conference call in February.
Besides Xyrem, Jazz’s narcolepsy portfolio also includes Sunosi, which launched a year ago. In the first quarter, Sunosi registered sales of $1.9 million, a steep drop from the $2.7 million it tallied in the fourth quarter of 2019. Jazz attributed the decline to higher gross-to-net deductions from increased coupon utilization despite a total scripts increase of 41%.
Jazz plans to launch Xywav by the end of the year under a safety monitoring program that the FDA’s requiring because of risks of central nervous system depression and potential for abuse and misuse.
Jul 22, 2020
https://www.fiercepharma.com/



