Amneal Submits NDA for IPX203 for Parkinson’s Disease
Amneal Pharmaceuticals has submitted a New Drug Application (NDA) to the FDA for its investigational candidate IPX203 for the treatment of patients with Parkinson’s disease.
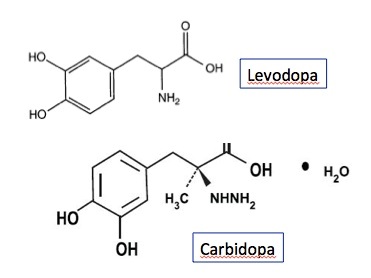
IPX203 is an oral formulation of carbidopa/levodopa extended-release capsules.
The submission is based on results from a phase 3 clinical trial demonstrating IPX203’s positive impact on periods when symptoms are better controlled. The investigational drug typically extended those periods by 1.55 more hours.
The company said its aim with IPX203 is to provide an option to patients with Parkinson’s disease that provides reduced dose frequency compared to immediate-release carbidopa/levodopa, the gold standard for treatment since the 1970s.
Parkinson’s is a neurological disorder characterized by slowness of movement, stiffness, resting tremor and impaired balance.
September 6, 2022



