Azurity Gets FDA Nod for Zonisade in Patients With Epilepsy
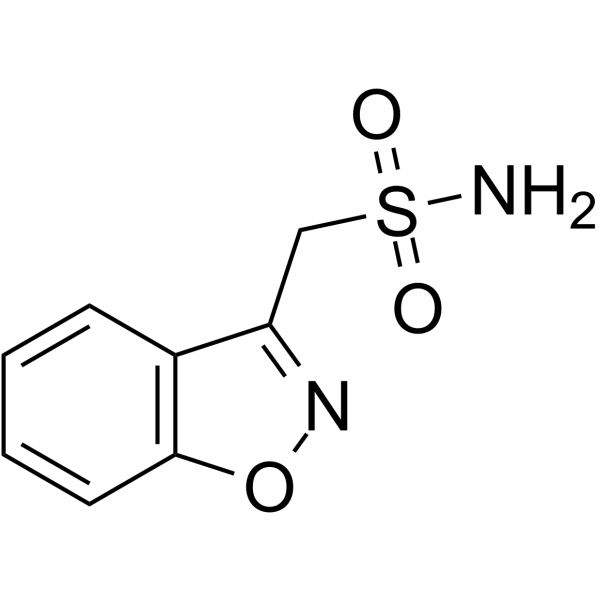
The FDA has approved Azurity Pharmaceuticals’ Zonisade (zonisamide oral suspension) as an adjunctive therapy for the treatment of partial seizures in epilepsy patients aged 16 years and older.
The approval makes Zonisade the only FDA-approved oral liquid formulation of zonisamide. The oral formulation will benefit patients who have difficulty swallowing or who are unable or unwilling to take tablets, the company said.
The drug’s efficacy and tolerability were established in three clinical trials, but not for individuals under 16 years old.
In a Complete Response Letter to Azurity in 2021, the FDA declined to approve the company’s New Drug Application because the agency was unable to inspect a third-party manufacturing facility due to travel restrictions.
Azurity Pharmaceuticals
Azurity Pharmaceuticals is a privately held, specialty pharmaceutical company that focuses on innovative products that meet the needs of patients with underserved conditions. As an industry leader in providing unique, accessible, and high-quality medications, Azurity leverages its integrated capabilities and vast partner network to continually expand its broad commercial product portfolio and robust late-stage pipeline. The company’s patient-centric products span the cardiovascular, neurology, endocrinology, gastro-intestinal, institutional, and orphan markets, and have benefited millions of patients.
July 20, 2022



