CRISPR Therapeutics Receives Regenerative Medicine Advanced Therapy Nod
CRISPR Therapeutics has received a Regenerative Medicine Advanced Therapy designation from the FDA for its CTX130 for treatment of patients with either of two types of cutaneous T-cell lymphoma — Mycosis Fungoides and Sézary Syndrome.
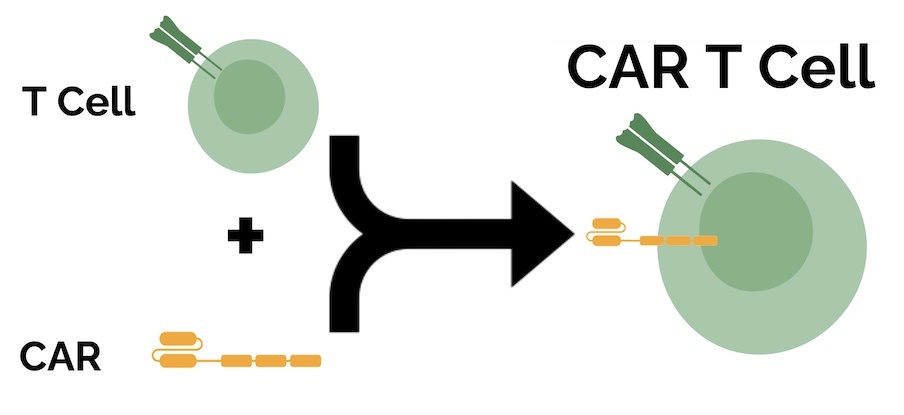
A CAR T cell therapy targeting the CD70 protein coding gene, CTX130 has also received FDA’s Orphan Drug designation from the FDA.
The therapy is currently being investigated in two ongoing phase 1 clinical trials. One study is evaluating the drug in relapsed or refractory T or B cell malignancies and the other trial is assessing it for relapsed or refractory renal cell carcinoma.
The FDA grants Regenerative Medicine Advanced Therapy status to cell therapies intended to treat, modify, reverse or cure a serious or life-threatening disease or condition. Sponsors must show preliminary clinical evidence indicating that the therapy has the potential to address unmet medical needs in a disease or condition.
About CRISPR Therapeutics
CRISPR Therapeutics AG is a Swiss–American biotechnology company headquartered in Zug, Switzerland. In fiscal year 2019, the company had revenues of $289.59 million, with net income of $66.86 million. In the same year, the number of employees stood at 304. As of December 2021, the company had a market capitalization of over $6 billion. CRISPR Therapeutics' investors include German chemical company Bayer. The company operates R&D in Cambridge, Massachusetts.
September 29, 2022



