EU Approves Kapruvia for CKD-Associated Pruritus
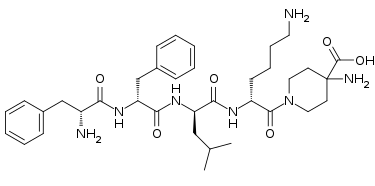
The European Commission has granted marketing authorization to Cara Therapeutics’ and Vifor Pharma’s Kapruvia (difelikefalin) for the treatment of patients with moderate-to-severe pruritus associated with chronic kidney disease (CKD) who are receiving hemodialysis, making it the first EU-approved therapy for the condition.
The EU approval was based on positive results from two phase 3 trials in which participants given the drug showed significant improvement in disease-associated itching.
Cara Therapeutics, the injectable drug’s developer, stands to earn up to $470 million in licensing and other payments from Vifor Pharma in connection with EU sales.
Kapruvia is marketed as Korsuva in the U.S. The FDA approved it for the same indication in August 2021.
April 29, 2022



