European Commission Greenlights Idorsia’s Insomnia Drug Quviviq
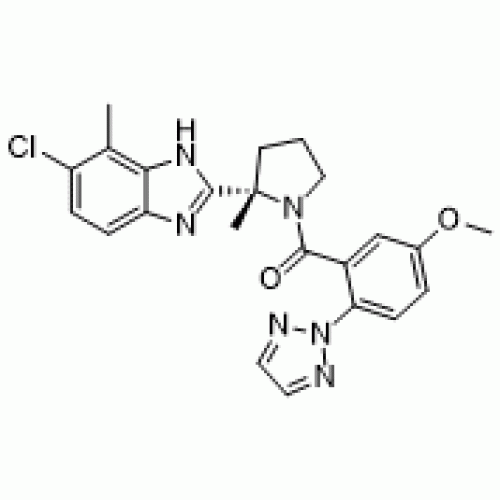
The European Commission has granted marketing authorization for Idorsia’s Quviviq (daridorexant) for the treatment of adult patients with chronic insomnia disorder.
Quviviq, which was approved in the U.S. in January, operates by blocking the binding of wake-promoting neuropeptides orexins.
In a study with 1,854 participants, 50 mg of Quviviq led to significant improvements in sleep onset, sleep maintenance and self-reported total sleep time compared to placebo.
Idorsia
The new company Idorsia was created from former Actelion drug discovery operations and early-stage clinical development assets and listed in June 2017 on the SIX Swiss Exchange.
The purpose of Idorsia is to discover, develop and bring more, innovative medicines to patients. Company have four phase 3 products: daridorexant (insomnia), clazosentan (treatment of vasospasm following a sub-arachnoid subarachnoid hemorrhage), aprocitentan (difficult to treat hypertension) and lucerastat (Fabry's disease). Pipeline has 12 assets in total.
May 4, 2022



