FDA and EMA Accept Pfizer Submissions for Ritlecitinib for Alopecia Areata
The FDA and EMA have accepted Pfizer’s New Drug Application (NDA) and Marketing Authorization Application (MAA) for ritlecitinib, both for adults and adolescents 12 years of age and older with alopecia areata, an autoimmune disease that leads to hair loss.
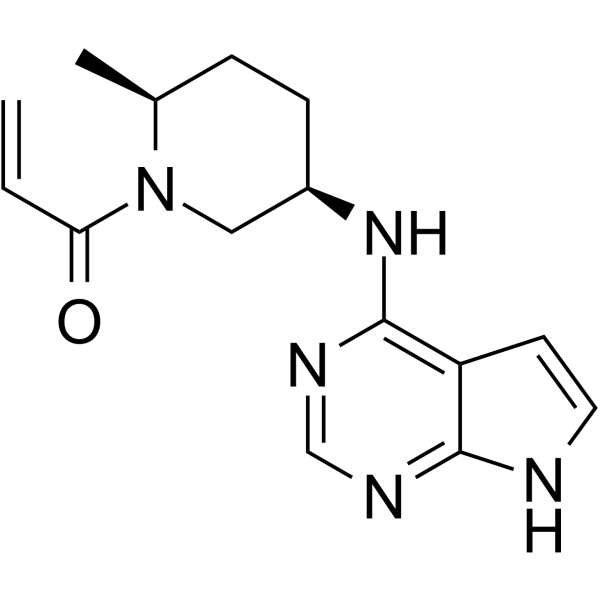
Ritlecitinib is an investigational once-daily pill that is a dual inhibitor of the TEC family of tyrosine kinases and of Janus kinase 3, the company said.
The submissions were based on results from a phase 2b/3 study and an ongoing phase 3 study. In the phase 2b/3, ritlecitinib demonstrated meaningful improvements in scalp hair regrowth, said the company.
The FDA is expected to make a decision on the NDA in the second quarter of 2023. The EMA’s decision is anticipated in the fourth quarter of 2023.
Ritlecitinib (PF-06651600) is an orally active and selective JAK3 inhibitor with an IC50 of 33.1 nM.
September 13, 2022



