FDA Approves Heron’s Aponvie for Postoperative Nausea
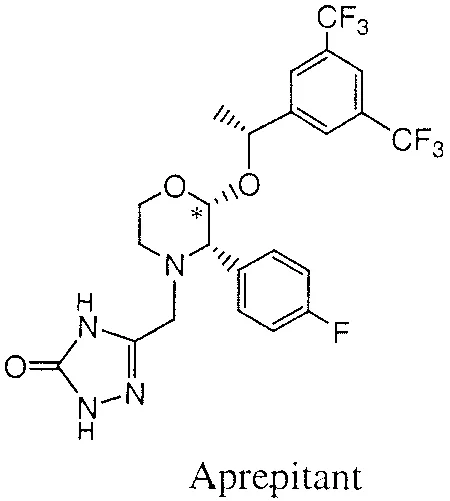
The FDA has approved Heron Therapeutics’ Aponvie (aprepitant) for the prevention of postoperative nausea and vomiting (PONV), common adverse effects of anesthesia and surgery.
An injectable emulsion that is indicated for intravenous use, Aponvie is a neurokinin-1 (NK1) receptor antagonist belonging to a class of antiemetic drugs.
Results from two clinical studies supported the agency approval. Treatment with aprepitant resulted in approximately 50 percent fewer patients vomiting in the first 48 hours compared to another antiemetic, ondansetron, the company said.
Aponvie will offer an option for the approximately 36 million patients undergoing surgical procedures in the country each year with high to moderate risk for PONV, Heron said.
Heron (HRTX) is a commercial-stage biotechnology company focused on improving the lives of patients by developing best-in-class treatments that address some of the most important unmet patient needs. Heron Therapeutics is developing novel, patient-focused solutions that apply its innovative science and technologies to already approved pharmacological agents for patients suffering from cancer or pain.
September 21, 2022



