Kintara Therapeutics gains after FDA fast track nod for brain cancer treatment VAL-083
Kintara Therapeutics (NASDAQ:KTRA) on Wednesday said that the U.S. FDA had granted a Fast Track designation to its therapeutic VAL-083 for the treatment of newly-diagnosed unmethylated glioblastoma (GBM), the most aggressive form of brain cancer.
Shares of the micro-cap biopharmaceutical company jumped ~25% to $0.23 in premarket trading on the news.
The FDA's Fast Track approval is a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
KTRA expects to announce data from its international phase 2/3 GBM AGILE study around the end of 2023.
Kintara Therapeutics, Inc
Kintara Therapeutics is dedicated to the development of novel cancer therapies for patients who are failing or resistant to current treatment regimens.
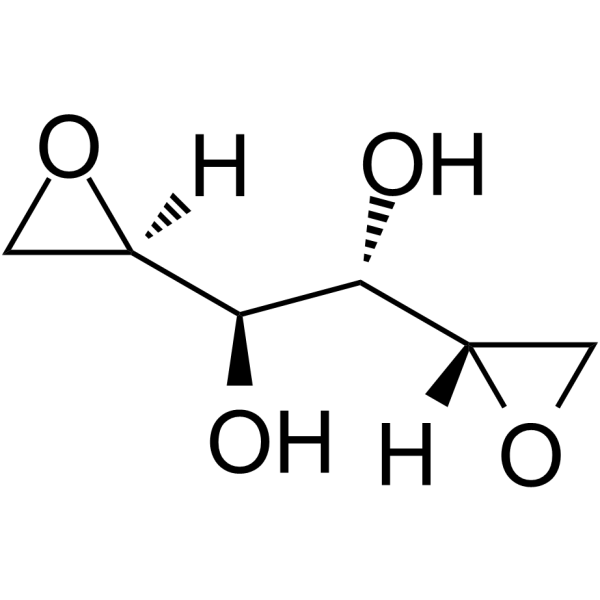
VAL-083 (Synonyms: Dianhydrodulcitol; Dianhydrogalactitol)
VAL-083 is a bi-functional DNA-targeting agent which preclinically demonstrated activity against multiple solid and hematologic tumors, including brain, cervical, and ovarian. The agent was granted orphan drug designations by the FDA for the treatment of GBM, medulloblastoma, and ovarian cancer. VAL-083 is an alkylating agent that creates N7 methylation on DNA, with antitumor activity.
Jun 16, 2022



