Pfizer Recalls Blood Pressure and Heart Failure Drug Accupril Due to Nitrosamine Content
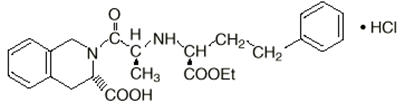
Pfizer has recalled five lots of Accupril (Quinapril HCl), its blood pressure adjunctive therapy for heart failure, due to the presence of unacceptable levels of a nitrosamine.
The company said its testing showed that the levels of the nitrosamine N-nitroso-quinapril were above the acceptable daily intake level, which presents an increased risk to patients of developing cancer over time.
The five affected lots were distributed within the U.S. and Puerto Rico to wholesalers and distributors between December 2019 and April 2022. The affected lots include the 10-mg, 20-mg and 40-mg doses of the drug.
Pfizer said that no adverse events have been reported relating to the recalled products.
April 26, 2022
https://www.fdanews.com/



