Pfizer Recalls Blood Pressure Drug Accuretic in UK Due to Nitrosamine Risk
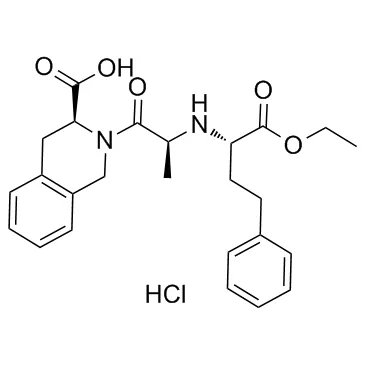
Quinapril hydrochloride
Pfizer has recalled a single batch of its blood pressure drug, Accuretic (quinapril HCl/hydrochlorothiazide), in the UK following the detection of a nitrosamine contaminant, N-nitroso-quinapril, at levels above the acceptable limit.
The batch of concern, numbered DD4842, was for quinapril HCl 10 mg/ hydrochlorothiazide 12.5 mg film-coated tablets distributed in April 2020.
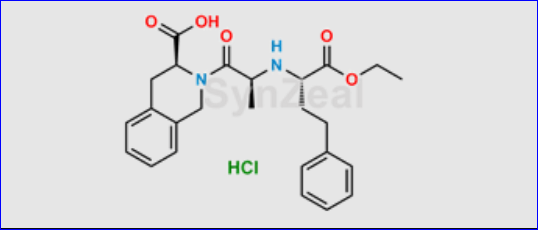
N-nitroso-quinapril
Consumption of nitrosamine at amounts above acceptable levels long-term could lead to an increased risk of cancer.
The recall will affect Accuretic supply at the wholesaler and pharmacy levels, the company said.
Accuretic is a prescription medicine used to treat the symptoms of High Blood Pressure (Hypertension). Accuretic may be used alone or with other medications.
Accuretic belongs to a class of drugs called ACEI/Diuretic Combos; ACEI/HCTZ Combos.
It is not known if Accuretic is safe and effective in children younger than 18 years of age.
April 4, 2022



