Real-World Data Support the Use of Sobi and Sanofi’s Elocta for Hemophilia A
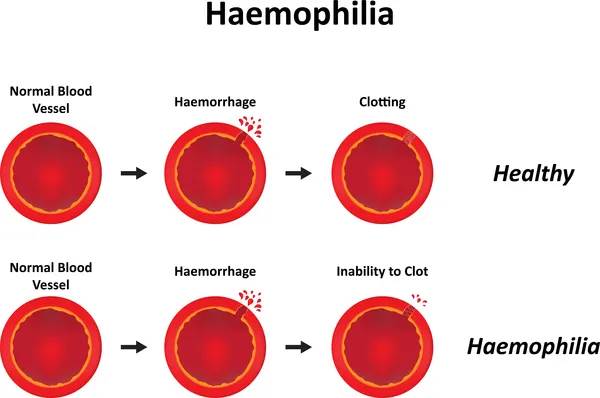
Sobi and Sanofi’s Elocta (efmoroctocog alfa) has shown efficacy in a real-world study in patients with the rare genetic bleeding disorder hemophilia A.
Elocta met three primary endpoints, including reductions in annual bleeding rates, annualized injection frequency and annualized Factor VIII consumption, according to data reported at the International Society on Thrombosis and Hemostasis Congress in London.
“These new data further expand the already extensive body of evidence supporting Elocta’s potential to elevate protection for people with hemophilia A,” said Anders Ullman, Sobi’s chief medical officer.
Elocta, which is known as Eloctate in the U.S., was approved by the FDA in 2014 for treatment of adults and children with hemophilia A.
July 15, 2022



