Roche Posts Positive Results for Evrysdi in Type 2 and 3 Spinal Muscular Atrophy
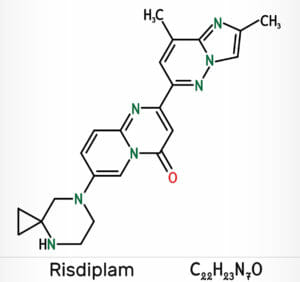
Roche has published long-term data confirming the efficacy and safety of its drug Evrysdi (risdiplam) in patients age two to 25 who have type 2 or type 3 spinal muscular atrophy, a rare and often fatal genetic disease affecting muscle strength and movement.
Patients taking Evrysdi were more likely to have improved or stable motor function measure scores than the untreated group and increases in motor function were sustained at three years while adverse events decreased over the same period, the company said.
Roche also announced that interim results from its trial of Evrysdi in infants demonstrated that the majority of babies treated with the drug for at least 12 months were able to stand and walk within timeframes typical of healthy babies.
The FDA approved Evrysdi to treat patients two months and older with Type 1 spinal muscular atrophy in August 2020, making it the first oral drug approved to treat the disease.
March 17, 2022



