The US approved the ecstasy test for PTSD
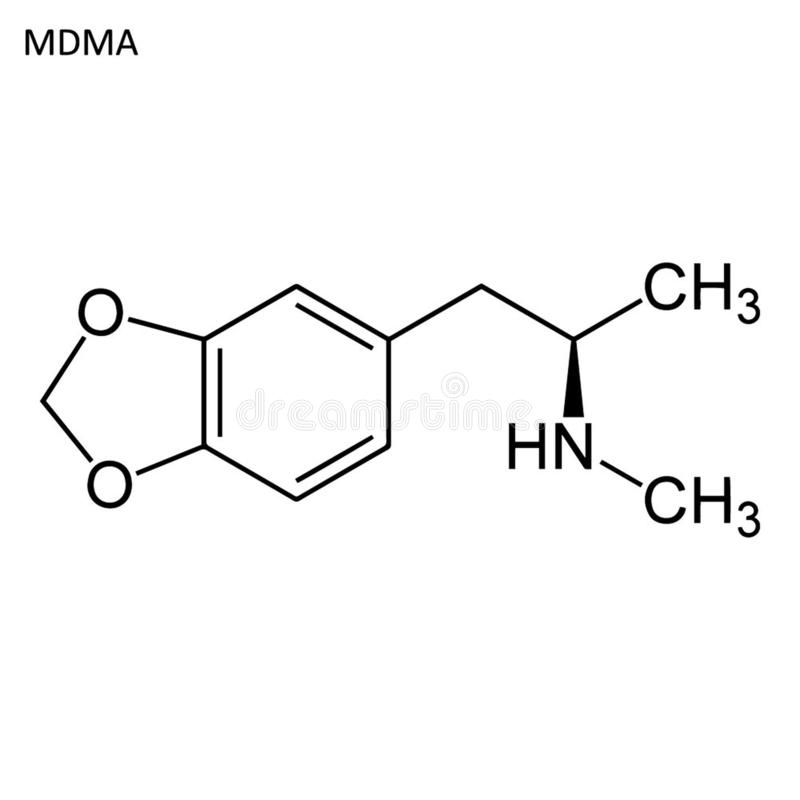
The Interdisciplinary Association for Psychedelic Research reports that ecstasy (3,4-methylenedioxymethamphetamine, MDMA) will be used for patients suffering from post-traumatic stress disorder (PTSD).
The Food and Drug Administration (FDA) has approved a trial, a group psychotherapy session that will use ecstasy . This is stated in a press release from the Interdisciplinary Association for Psychedelic Studies (MAPS).
It was noticed that for the first time trials using these pills as medicine began in the United States in 2016. In just one year, doctors took great steps and gave this treatment the status of a success therapy – this is the name of experimental treatments that are planned to introduce into medical practice.
In 2020, before the end of the extensive trials, the FDA issued special authorization for the treatment of 50 patients with MDMA. In May 2021, phase III trials were successfully completed, which could serve as the basis for the legal sale of MDMA.
The department added that it was about single therapy, but now they want to take ecstasy for group therapy, because they are cheaper and common.
According to future head of testing Chris Stoffer, he’s not primarily interested in the potential wide availability, but in the expected efficiency.
“I am motivated for this work by the unique therapeutic possibilities of group cohesion that I have observed in previous experiments with oxytocin and psilocybin,” he said.
It was previously reported that a single injection of psychedelic psilocybin increases the number of synapses in the brain and decreases the number of serotonin 5-HT2A receptors.
JULY 6, 2022



