Timber Pharmaceuticals Announces Successful End-of-Phase 2 Meeting with FDA for TMB-001 in Moderate to Severe Congenital Ichthyosis
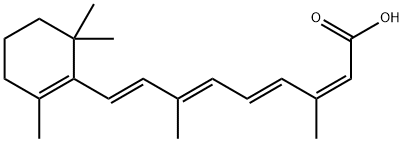
Timber Pharmaceuticals announced the successful completion of an End-of-Phase 2 meeting with the U.S. FDA resulting in a clear path towards a pivotal Phase 3 study for its lead asset, TMB-001, a topical isotretinoin formulated using the company’s patented IPEG™ delivery system.
The company plans to initiate a Phase 3 study in the second quarter of 2022 in patients with moderate to severe congenital ichthyosis (CI).
Timber completed the Phase 2b CONTROL study evaluating TMB-001 in moderate to severe congenital ichthyosis (CI) in September 2021. Full data was subsequently presented at the 2022 Winter Clinical Dermatology Conference held January 14–19, 2022 in Koloa, Hawaii.
The randomized, parallel, double-blind, vehicle-controlled study was designed to evaluate the efficacy and safety of two concentrations of TMB-001 (0.05%, 0.1%) versus vehicle administered twice daily in patients (n=33) with moderate to severe subtypes of CI, including X-linked ichthyosis (XLRI) and autosomal recessive congenital ichthyosis (ARCI), which includes lamellar ichthyosis (LI). The treatment duration was 12 weeks.
February 3, 2022



