Two FDA Committees to Discuss Perrigo’s Opill Drug for OTC Use
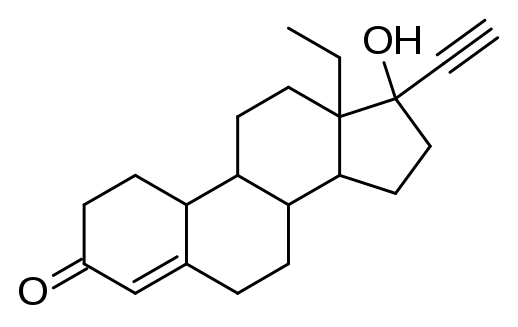
Two FDA committees, the Nonprescription Drugs Advisory Committee and the Obstetrics, Reproductive, and Urologic Drugs Advisory Committee, will be reviewing Perrigo’s Opill (norgestrel tablets) for over-the-counter (OTC) use.
Perrigo’s affiliate HRA Pharma filed the application in July for this switch from prescription to OTC.
The progestin-only daily birth control drug was first approved by the FDA in 1973, but patients could only access the drug through a prescription. If this application is green lighted, Opill will be the first daily birth control pill available in an OTC format.
The two committees are expected to meet on Nov. 18 to review the company’s application.
Perrigo Company plc
Perrigo Company plc is an American Irish–registered manufacturer of private label over-the-counter pharmaceuticals, and while 70% of Perrigo's net sales are from the U.S. healthcare system, Perrigo is legally headquartered in Ireland for tax purposes, which accounts for 0.60% of net sales. Perrigo engages in the acquisition (for repricing), manufacture, and sale of consumer healthcare products, generic prescription drugs, and active pharmaceutical ingredients (APIs), primarily in the United States, from its base in Ireland.
September 13, 2022



