FDA Extends Ibuprofen Suspension Guidance
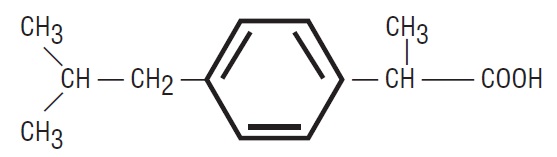
The FDA has updated its guidance on the compounding of ibuprofen oral suspension products to include state-licensed pharmacies and federal facilities.
The original guidance — issued in January to respond to a record high demand for such products due to COVID-19, respiratory syncytial virus and influenza — allowed outsourcing facilities to compound ibuprofen for use in hospitals and health systems.
The guidance sets out the conditions under which the FDA generally does not intend to take regulatory action regarding stability testing and expiration date requirements.
February 13, 2023
https://www.fdanews.com/
The original guidance — issued in January to respond to a record high demand for such products due to COVID-19, respiratory syncytial virus and influenza — allowed outsourcing facilities to compound ibuprofen for use in hospitals and health systems.
The guidance sets out the conditions under which the FDA generally does not intend to take regulatory action regarding stability testing and expiration date requirements.
February 13, 2023
https://www.fdanews.com/



