FDA Grants Accelerated Approval to Eli Lilly’s Jaypirca in Mantle Cell Lymphoma
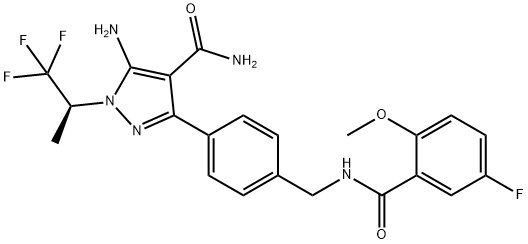
Eli Lilly’s Jaypirca (pirtobrutinib) has gained FDA accelerated approval for treatment of adult patients with relapsed or resistant mantle cell lymphoma after at least two lines of systemic therapy including a Bruton’s tyrosine kinase inhibitor.
The agency decision was based on positive results in a phase 1/2 study in which participants had an overall response rate of 50 percent and a median duration of response of 8.3 months.
The kinase inhibitor oral drug will be made available in the U.S. in the coming weeks, the company said.
Under the accelerated approval terms, continued approval may be contingent on a confirmatory trial for verification of the treatment’s clinical benefit.
January 31, 2023



