FDA’s approval of Friedreich’s ataxia drug is a game changer for treatment of rare diseases
Reata Pharmaceuticals is aiming to make the drug omaveloxolone available by the second quarter of 2023.
A drug to treat a rare neurological disorder has only had to jump through one hoop—read: clinical trial—instead of the recommended two to win regulatory approval.
The US Food and Drug Administration (FDA) approved Reata Pharmaceuticals’ Skyclarys, the commercial name for the drug omaveloxolone, for the treatment of Friedreich’s ataxia in adults and adolescents aged 16 years and older yesterday (Feb. 28). Typically, the FDA only approves drugs after two successful final-phase, placebo-controlled studies. Reata’s approval is the first-of-its-kind in that it is based on just a single study, one focusing on the same set of patients over time rather than new ones—a potential game changer for the treatment of diseases that affect relatively few people
In 2020, the FDA had said there wasn’t enough evidence to greenlight the drug. Reata didn’t conduct another study citing lack of participants—very few people have the condition and fewer among them qualified for trial—but it did provide new data of long-term findings, showing patients who took the drug experienced an improvement in neurological functions such as speaking, swallowing and standing, compared to a placebo.
“The approval of Skyclarys, the first therapy specifically indicated for the treatment of Friedreich’s ataxia, is an important milestone for patients affected by this disease as well as their families and caregivers,” said Reata CEO Warren Huff in a press release. “We look forward to delivering Skyclarys to eligible patients as quickly as possible.”
The Texas-based biopharmaceutical company expects to complete the manufacturing and supply of Skyclarys—its first approved product—by the second quarter of this year.
What is Friedreich’s ataxia?
Friedreich’s ataxia is a rare genetic disease that causes progressive damage to the nervous system and creates movement problems including trouble walking, fatigue, changes in sensation, and slowed speech. It’s caused by a gene defect inherited from both parents. Symptoms often begin in late childhood and tend to get worse over time. It “commonly progresses to motor incapacitation and wheelchair reliance by their teens or early twenties, and eventually death,” according to Reata.
Currently, there is no cure.
What is Skyclarys?
The treatment is an oral, once-daily medication indicated for the treatment of Friedreich’s ataxia in adults and adolescents aged 16 years and older in the US. The recommended dosage is 150 mg, taken in three 50 mg capsules. It is supposed to be administered on an empty stomach, an hour before eating.
The FDA neuroscience chief is leaving the agency. Billy Dunn joined the FDA back in 2005 and climbed the ranks to eventually help set up and head the neuroscience office.
In recent years, Dunn came to be known as a controversial risk-taker. He pushed for the approval of other drugs for neurological conditions based on limited data, such as Biogen’s Alzheimer’s drug Aduhelm in 2021 and Amylyx’s ALS drug last year. His involvement in the Skyclarys decision likely nudged its approval over the finish line.
“He has made a major impact in how drugs are developed for a range of serious neurological diseases—many that previously had only limited treatment options,” Peter Stein, head of the regulator’s office of new drugs, wrote in an email to staff on Monday (Feb. 27), informing them of Dunn’s immediate departure.
News of his exit, which came before the FDA’s approval of Skyclarys was announced, caused Reata’s stock to drop 30%. The plunge “isn’t surprising given the nearly unanimous view on the street that [Reata’s drug] wouldn’t stand a snowball’s chance under the review of any other Officer/Director at FDA,” Baird analyst Brian Skorney wrote in a note that day.
Treating Friedreich’s ataxia, by the digits
5,000: Number of patients diagnosed with Friedreich’s ataxia in the US
150%: How much Reata’s stock jumped in after-hours trading on the news of Skyclarys’ FDA approval.
74,000: Signatories on a petition created by the Friedreich’s Ataxia Research Alliance (FARA), urging Reata to seek the treatment’s approval and for the FDA to consider its merit
$400 million: Projected US sales of Skyclarys by 2030, according to Jefferies analyst Maury Raycroft
$425,000: Cost of the drug per patient annually, as per Raycroft
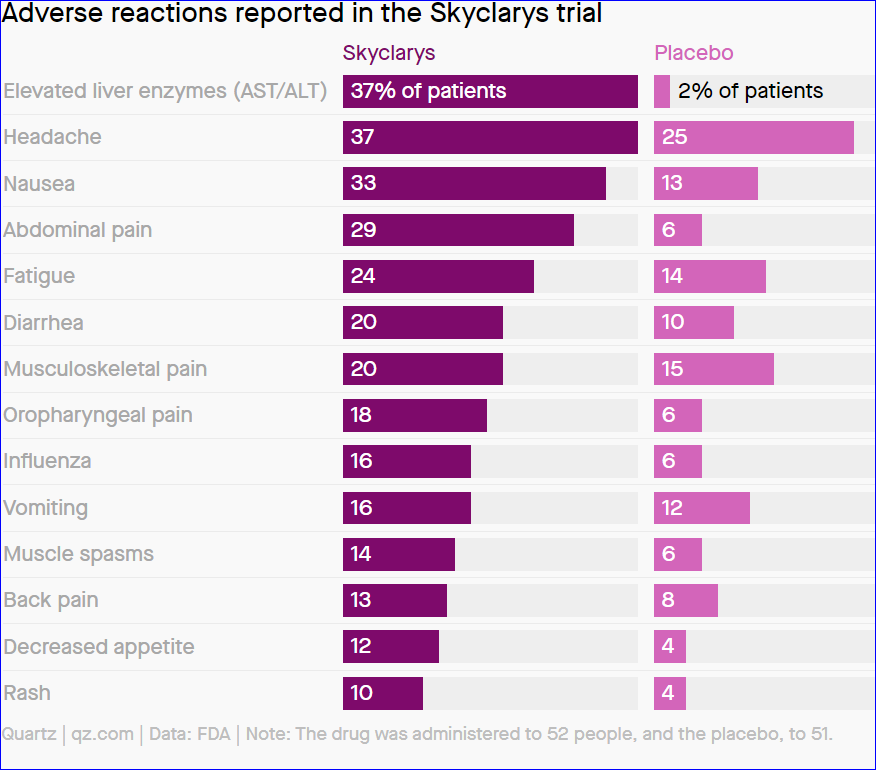
10%: Share of trial patients who showed adverse reactions, mostly mild or moderate in severity.
Throwback: Reata’s other drug that the FDA did not approve
In December 2021, Reata’s chronic kidney disease drug was rejected 13-0 by a panel of FDA advisors, who concluded that the drug was not effective in slowing the progression of the condition and that its risks outweigh its benefits.
Published March 1, 2023



