Jazz Pharmaceuticals Loses REMS Patent Appeal
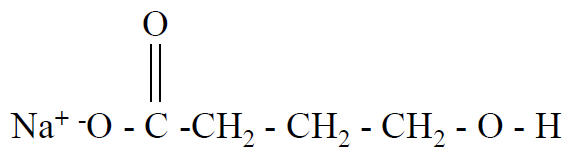
A federal appeals court has rejected Jazz Pharmaceuticals’ attempt to overturn a lower court ruling invalidating a patent claim covering the company’s Risk Evaluation and Mitigation Strategies (REMS) distribution system for its blockbuster narcolepsy therapy Xyrem (oxybate).
The case dates to July 2022 when Jazz sued Avadel Pharmaceuticals over the company’s new drug filing for a rival narcolepsy drug, Lumryz (sodium oxybate), which Jazz claimed infringed on its REMS patent protections for Xyrem.
The FDA requires a REMS for drugs with a high abuse potential — such as oxybate, which depresses the central nervous system. But Avadel maintains that Lumryz would be distributed under a decentralized REMS using multiple databases and pharmacies, with no overlap with the Jazz’s REMS system, which relies on a single, centralized pharmacy and database.
In November, the U.S. District Court for the District of Delaware ruled against Jazz, saying the company had wrongly used its REMS patent protections.
Jazz appealed the district court decision, prompting the U.S. Court of Appeals for the Federal Circuit to issue a ruling last week in favor of Avadel.
About Sodium oxybate
Sodium oxybate, sold under the brand name Xyrem among others, is a medication used to treat two symptoms of narcolepsy: sudden muscle weakness and excessive daytime sleepiness. It is used sometimes in France and Italy as an anesthetic given intravenously it is also used in Italy to treat alcohol addiction and alcohol withdrawal syndrome.Sodium oxybate is the sodium salt of γ-hydroxybutyric acid (GHB).
About Jazz Pharmaceuticals plc
Jazz Pharmaceuticals plc (a merger of Jazz Pharmaceuticals, Inc. and Azur Pharma plc) is a biopharmaceutical company based in Ireland. It was founded in 2003. One of the company's considerable products is the United States Food and Drug Administration (FDA) approved drug Xyrem (sodium oxybate), the sodium salt of the naturally occurring neurotransmitter γ-Hydroxybutyric acid (GHB). In 2017, net product sales of Xyrem were $1.187 billion, which represented 74% of the company's total net product sales. In 2019, Jazz was granted FDA-approval to market Sunosi with indications for treating excessive daytime sleepiness (EDS) in narcolepsy as well as obstructive sleep apnea (OSA). In 2022, it was announced that Axsome Therapeutics would be acquiring Sunosi from Jazz Pharmaceuticals. In 2007, the company pled guilty to felony charges related to its illegal marketing of Xyrem for off-label use. The company is also a member of the Pharmaceutical Research and Manufacturers of America (PhRMA).
March 2, 2023



