Lagevrio Disappoints in Phase 3 study for Post-Exposure Prevention of COVID-19
Merck’s Lagevrio (molnupiravir) has not demonstrated a statistically significant reduction in the risk of COVID-19 infection after household exposure to another person who has COVID-19.
In a phase 3 study, the drug did not achieve the primary endpoint of prevention, although participants taking Lagevrio were 23.6 percent less likely to develop COVID-19 through day 14 compared to those taking placebo.
Merck said the prevention study results “do not impact the efficacy and safety data observed” in the company’s phase 3 MOVe-OUT trial for the treatment of mild-to-moderate COVID-19.
Lagevrio is authorized by the FDA for the treatment of mild-to-moderate COVID-19 adult patients who are at high risk of progression to severe COVID-19.
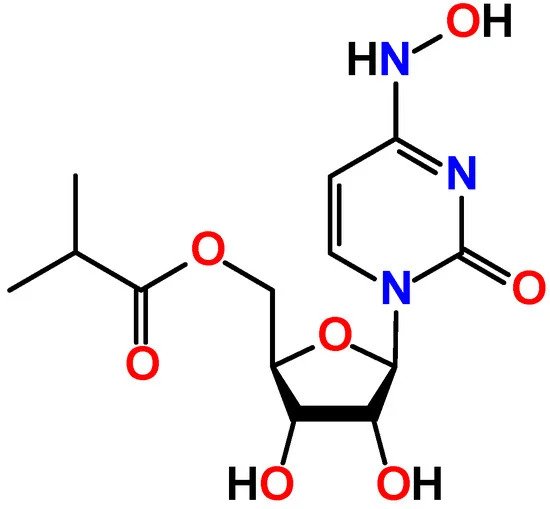
Molnupiravir, sold under the brand name Lagevrio, is an antiviral medication that inhibits the replication of certain RNA viruses. It is used to treat COVID-19 in those infected by SARS-CoV-2. It is taken by mouth. Molnupiravir is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine and exerts its antiviral action by introducing copying errors during viral RNA replication. Molnupiravir was originally developed to treat influenza at Emory University by the university's drug innovation company, Drug Innovation Ventures at Emory (DRIVE), but was reportedly abandoned for mutagenicity concerns. It was then acquired by Miami-based company Ridgeback Biotherapeutics, which later partnered with Merck & Co. to develop the drug further.
February 23, 2023



