Ascentage Pharma’s olverembatinib approved in Macau for leukaemia
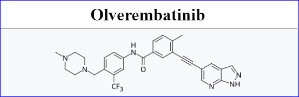
Ascentage Pharma has obtained approval for its new BCR-ABL1 [a gene sequence found in certain forms of leukaemia] tyrosine kinase inhibitor (TKI), olverembatinib, from the Pharmaceutical Administration Bureau of the Macau Special Administrative Region of the People’s Republic of China to treat haematological malignancy indications.
Indications for olverembatinib include use in adults with chronic-phase chronic myeloid leukaemia (CML-CP) or accelerated-phase CML (CML-AP) resistant to TKIs and harbouring the T315I mutation.
It is also indicated for adults with CML-CP who are resistant to or intolerant of first and second-generation TKIs. The development in Macau follows approvals on the Chinese mainland for the same indications.
Olverembatinib is the first third-generation BCR-ABL1 inhibitor approved by China’s National Medical Products Administration. It was developed by Ascentage Pharma with support from the National Major New Drug Development programme. The asset targets BCR-ABL1 and a range of BCR-ABL1 mutants, including the T315I mutation.
Its clinical trial results have been incorporated into the National Comprehensive Cancer Network guidelines for CML management. The commercialisation of olverembatinib in China is a joint effort between Ascentage Pharma and Innovent Biologics.
In June 2024, Ascentage Pharma and Takeda signed an option agreement to enter into an exclusive licence agreement for olverembatinib for CML and other haematological cancers.
July 10, 2024



