Bristol Myers Squibb’s Augtyro granted expanded FDA approval for solid tumours
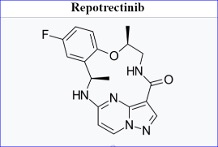
Bristol Myers Squibb’s Augtyro (repotrectinib) has been granted accelerated approval by the US Food and Drug Administration (FDA) to treat neurotrophic tyrosine receptor kinase (NTRK)-positive tumours.
The tyrosine kinase inhibitor (TKI) has been specifically authorised for use in adult and paediatric patients aged 12 years of age and older with tumours that are locally advanced or metastatic, or where surgical resection is likely to result in severe morbidity, who have progressed following treatment or have no satisfactory alternative therapy.
Augtyro, which was added to BMS’ oncology portfolio in 2022 through its $4.1bn acquisition of Turning Point Therapeutics, was approved by the FDA last November to treat adult patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer (NSCLC).
The FDA’s latest decision on the drug was supported by positive results from the phase 1/2 TRIDENT-1 study, which evaluated Augtyro in adults with NTRK-positive solid tumours. At a median follow-up of 17.8 months, 58% of Augtyro-treated patients who had not previously received any TKI treatment had a confirmed objective response rate. In TKI-pretreated patients, with a median follow-up of 20.1 months, the confirmed objective response rate was 50%. After one year of Augtyro treatment, 83% of TKI-naïve responding patients and 42% of TKI-pretreated responding patients were still in response.
The decision comes just two weeks after the FDA approved BMS’ CAR T cell therapy Breyanzi (lisocabtagene maraleucel) to treat adults with relapsed or refractory mantle cell lymphoma who have received at least two prior lines of systemic therapy, including a Bruton tyrosine kinase inhibitor.
The company also recently received approval from the European Commission for Opdivo (nivolumab) as part of a combination treatment for adults with unresectable or metastatic urothelial carcinoma.
June 17, 2024



