Eli Lilly's Obesity Drug Shows a Heart Benefit. Is It Time to Buy?
Shares of Eli Lilly notched a 3.5% gain on Thursday, Aug. 1, after the company reported positive clinical trial results for its increasingly successful weight management treatment. It looks like certain patients treated with tirzepatide were significantly less likely to suffer cardiovascular death and other heart failure outcomes compared to patients who were randomized to receive a placebo.
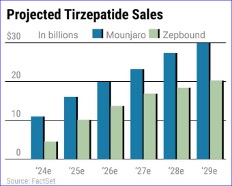
Tirzepatide is already marketed as Zepbound for weight management and Mounjaro for diabetes. Sales have been rocketing higher but so has Eli Lilly's stock price. To find out whether Eli Lilly stock is a good investment now, let's start by looking at what its new lead drug accomplished for heart failure patients.
If you only glanced at the headlines concerning Eli Lilly's latest clinical trial success, you might think tirzepatide has been proven to prevent heart attacks and strokes across the board, but that isn't the case. The phase 3 Summit trial evaluated the drug in adults with obesity and a complex condition called heart failure with preserved ejection fraction or HFpEF. This is a condition where the heart beats normally but still can't pump enough blood because it isn't filling up before it contracts.
An endless array of conditions can prevent hearts from filling properly. Among roughly 3 million Americans with HFpEF at the moment, age, obesity, and diabetes are some of the most commonly noted risk factors.
Eli Lilly hasn't figured out how to reverse the aging process, but tirzepatide is a leading treatment for both type 2 diabetes and weight management. The Summit trial enrolled 731 patients with both HFpEF and obesity, many who also have diabetes, and randomized them to receive tirzepatide or a placebo. After following those patients for two years, investigators measured a 38% risk reduction regarding heart failure outcomes such as hospitalization and cardiovascular death for patients who received tirzepatide.
In addition to a much lower risk of heart failure outcomes, patients given tirzepatide also reduced their weight by 15.7% on average compared to a 2.2% average weight reduction for patients given a placebo. Later this year, Lilly will submit Summit trial results to the Food and Drug Administration (FDA). The side effects measured in the Summit trial were on par with those seen in previous studies that led to tirzepatide's approval to treat diabetes and obesity.
August 7, 2024



