FDA Takes Step Toward Removal of Ineffective Decongestants From the Market
FDA Takes Step Toward Removal of Ineffective Decongestants From the Market
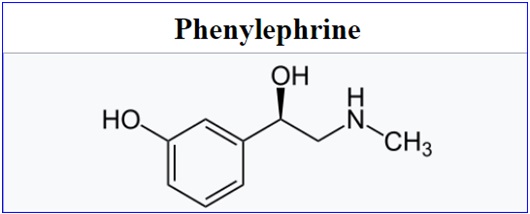
The FDA has proposed removing oral phenylephrine from its guidelines for over-the-counter drugs due to inefficacy as a decongestant. Use of this ingredient in cold and allergy medicines grew after a federal law required that pseudoephedrine-containing products be kept behind pharmacy counters.
A pharmaceutical ingredient that’s been a key component in nasal decongestants for decades is having its efficacy called into question, and the FDA is now weighing whether to change the compound’s regulatory status — potentially leading to many widely used over-the-counter cold and allergy products being pulled from pharmacy shelves. But before the FDA takes any action, it wants to hear what the public thinks.
The ingredient, oral phenylephrine, by itself and in combination with other active ingredients, is in decongestants sold under numerous brand names, such as Sudafed and Mucinex. All products containing oral phenylephrine continue to be available to consumers for now. But on Thursday, the FDA proposed an order that would remove oral phenylephrine from its non-prescription drugs guidelines, called the OTC Monograph. The regulator emphasized that this proposed order is due to lack of efficacy, not because of safety.
At one time, phenylephrine was rare in decongestants because pseudoephedrine was the preferred active ingredient. But that compound can be made into methamphetamine. Efforts to curb meth production led to the Combat Methamphetamine Act of 2005, which moved pseudoephedrine-containing drugs behind pharmacy counters. Drugmakers responded by making more products with oral phenylephrine, which is not covered by this law.
Last year, an FDA advisory committee discussed phenylephrine and unanimously voted that current scientific data do not support the ingredient’s efficacy as a nasal decongestant.
Phenylephrine is also an ingredient in some nasal spray decongestants. The FDA’s proposal does not extend to such products.