India’s DCGI approves Orchid Pharma’s API Enmetazobactam
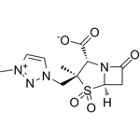
Orchid Pharma has secured approval from the Drugs Controller General of India (DCGI) to manufacture and market its new chemical entity (NCE), the active pharmaceutical ingredient (API) Enmetazobactam.
The DCGI also approved the production and marketing of the finished dosage form (FDF) of Cefepime and Enmetazobactam as a dry powder injectable. The antibiotic drug combination is specifically indicated for complicated urinary tract infections (cUTI), including the treatment of acute pyelonephritis.
Other indications comprise hospital-acquired pneumonia (HAP), ventilator-associated pneumonia, and bacteremia when associated with or suspected to be linked to either cUTI or HAP.
Orchid Pharma’s new combination drug addresses the urgent need for effective treatments against severe infections caused by resistant bacteria, a critical concern in the global health community’s fight against antimicrobial resistance (AMR).
The United Nations and the World Health Organization have declared AMR to be a “silent pandemic”, attributing to five million deaths in 2019.
The company anticipates a successful launch and distribution of Enmetazobactam and its combination with Cefepime, aiming to enhance the treatment options available for serious infections in the country.
Orchid Pharma is the only Indian pharmaceutical company to have invented an NCE.
June 10, 2024



