Lexicon to face FDA AdComm for Zynquista approval in diabetes and CKD
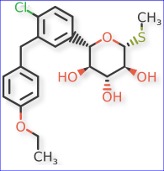
A FDA advisory committee (AdComm) is set to evaluate Lexicon’s Zynquista as an adjunct therapy for type 1 diabetes and chronic kidney disease.
It looks like Lexicon Pharmaceuticals’ second bid to get US Food and Drug Administration (FDA) approval for Zynquista (sotagliflozin) will be as arduous as the first go around, as the regulatory agency has convened an advisory committee (AdComm) to evaluate its efficacy.
The FDA’s Endocrinologic and Metabolic Drugs AdComm will discuss the benefit/risk profile of Zynquista as an adjunct to insulin therapy for glycaemic control in adults with type 1 diabetes (T1D) and chronic kidney disease (CKD). The AdComm is set to meet on 31 October 2024.
Lexicon added that convening of the AdComm will not affect the Prescription Drug User Fee Act (PDUFA) goal date of 20 December 2024 set by the FDA. An AdComm is convened upon request of the FDA to review and evaluate the safety and efficacy data of a therapy. However, the AdComm recommendations are non-binding, with the final decision made by the FDA.
The US-based company does not have a good history with the AdComm regarding Zynquista. In 2019, the FDA declined to approve the therapy citing safety concerns following a split 8-8 vote from its AdComm. The AdComm was particularly concerned about the risk of diabetic ketoacidosis, a serious complication which can become life-threatening if urgent treatment is not administered.
Although Lexicon was able to get approval for Zynquista as an adjunct treatment to insulin in T1D and CKD from the European Medicine Agency (EMA) in 2019. The company was not able to maintain the licensing agreement with Sanofi for Zynquista.
It has been a hard year for Lexicon. While the company has reaffirmed its commitment to support Zynquista’s development and approval. This month, it instituted cash-saving measures, including a 50% reduction in its “current field force”, which is expected to take place in Q3 this year.
Zynquista is an oral dual inhibitor of the sodium-dependent glucose co-transporter types 1 and 2 (SGLT1 and SGLT2), which play a role in glucose uptake and reabsorption. The drug was approved by the FDA to reduce the risk of cardiovascular death and heart failure. It was also approved for use in patients with type 2 diabetes, CKD, and other underlying cardiovascular risk factors. It is marketed as Inpefa for the cardiovascular indication.
Lexicon is also evaluating sotagliflozin as a treatment for hypertrophic cardiomyopathy. This month, the company initiated a placebo-controlled Phase III trial (NCT06481891) evaluating the drug in patients with symptomatic hypertrophic cardiomyopathy.



