The crowd and panel gave Gilead a standing ovation after the full data from the Phase III PURPOSE trial was announced at AIDS 2024
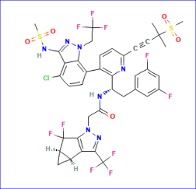
Gilead received a standing ovation after announcing its full, perfect efficacy data from the Phase III PURPOSE 1 trial of pre-exposure prophylaxis (PrEP) lenacapavir at the world’s largest HIV and AIDS conference.
On top of the data, the company also committed to prioritising swift access and enabling efficient paths for regulatory approval of lenacapavir for high-incidence, resource-limited countries.
Dr Linda-Gail Bekker, chief executive officer of the Desmond Tutu Health Foundation in South Africa, received a standing ovation from the crowd and other presenters, following her presentation of the data at the conference on 24 July.
Bekker presented the full data for the PURPOSE 1 trial (NCT04994509) which evaluated the PrEP lenacapavir versus alternative daily PrEP in 5,345 HIV-negative, adolescent girls and young women aged 16 to 26 in South Africa and Uganda.
The trial demonstrated zero infections in HIV prevention in women. Lenacapavir demonstrated superior prevention compared with once-daily oral Truvada and Descovy. A total of 16 incident HIV cases among 1,068 women were observed in the Truvada group while 39 incident HIV cases among 2,136 women were observed in the Descovy group.
Lenacapavir also had high rates of adherence, 91.5% at week 26 and 92.8% at one year. Daily oral adherence rates, measured through blood samples from a subset of patients, was low and declined over time. At the one-year point, 15.9% of Descovy patients recording high or medium adherence and 7% of Truvada patients reported high or medium adherence.
After meeting the primary endpoint, Gilead was able to lift the blinded phase of the PURPOSE 1 and offer open-label lenacapavir to all participants. As of July 23, more than 840 trial participants have already opted to switch to lenacapavir.
The safety profile remained good, with the most common adverse event being injection site reaction. Aside from injection site reactions, the most common AEs were headache, urinary tract infection, genitourinary chlamydia infection, and nausea.
July 26, 2024



