Allosteric inhibitors
Allosteric inhibitors and possibilities of their application
History of allostery
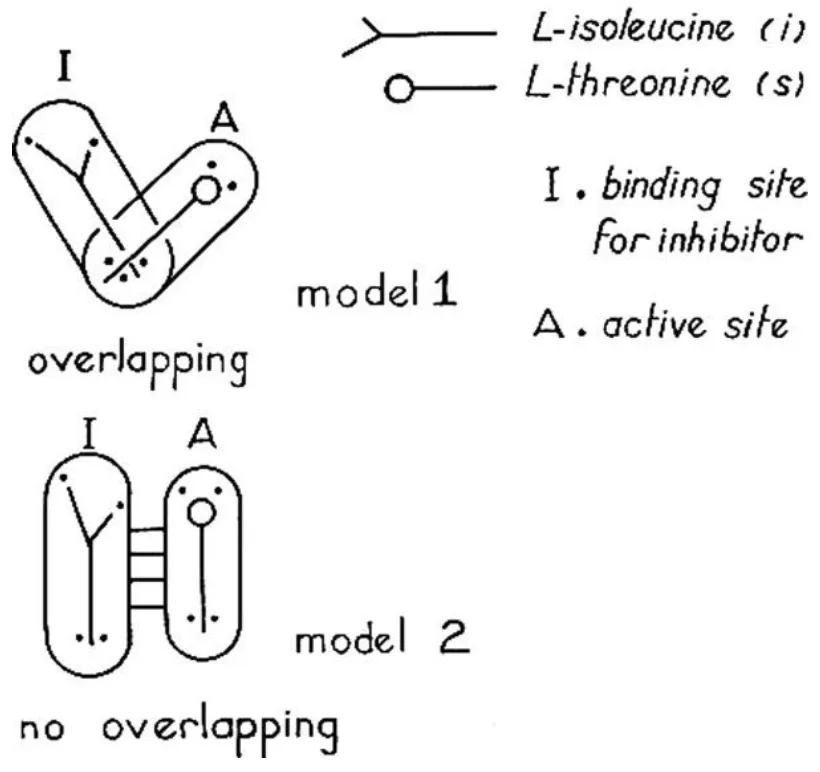
The first known discovery of a cooperative protein’s ligands attaching to different sites was made by Danish physician Christian Bohr in 1904. So-called “Bohr effect” is a phenomenon in which carbon dioxide affects interaction between hemoglobin and oxygen by linking to the specific site [1]. In 1961 Jacques Monod and Francois Jacob named this kind of regulation “allosteric” inhibition because of attaching to the distant area on the enzymatic surface rather than to its active site [2].
Figure 1. Two different models of L-threonine deaminase regulation by L-isoleucine, according to Jean-Pierre Changeux [2]. Second model described two distant sites – one for inhibitor and one for substrate. Later in their subsequent publication, Jacques Monod and Francois Jacob named this kind of regulation “allosteric”.
First drug based on an allosteric inhibitor was approved by the US FDA in 2004. Since then this “indirect” type of inhibition has been growing in popularity rapidly [3]. Here are several cases of allosteric inhibitors being used as drugs today:
- Diazepam – a medication used to treat anxiety, panic attacks, seizures, etc.;
- Maraviroc – an antiviral drug used to treat HIV infection;
- Cinacalcet – a medication used to treat parathyroid carcinoma, HPT.
Overview of inhibition
Enzyme, or protein, inhibitors are chemical compounds interfering with the process of catalysis and slowing enzymatic chemical changes. Prevalence of enzymatic reactions in cells is the reason for the popularity of inhibitors’ usage as drugs. Researchers distinguish two main classes of inhibitors. First is called reversible for slowing down a chemical reaction, while irreversible completely stops it.
Another inhibition classification is based on their interaction with an enzyme. According to it, inhibitors can be divided into three main groups: competitive, uncompetitive and mixed inhibitors (table 1).
Table 1. Three types of enzyme inhibition [4].
|
Type |
Competitive |
Uncompetitive |
Mixed |
|
Binds |
Enzyme |
Enzyme-substrate complex |
Enzyme or enzyme-substrate complex |
|
Site |
Orthosteric/allosteric |
Allosteric |
Allosteric |
|
Scheme |
|
|
|
Competitive inhibitors are in competition with substrate for the same area of interaction (so-called active site) on the enzymatic surface. Most of these inhibitors have a substrate-like molecular structure. However, the enzyme, forming a complex with an inhibitor, cannot catalyze chemical transformations as effectively as it would be without inhibition.
Uncompetitive and mixed inhibitors form bonds with distant site of the enzyme different from the active one substrate bonds with (it’s also called allosteric site). Uncompetitive inhibitors interact with distant site of an enzyme after the enzyme-substrate complex is formed. Mixed inhibitors, in contrast, can interact with complex as well as enzyme itself. An affinity for attaching to the enzyme with or without the substrate already bound to it varies depending on the inhibitor. Group of chemical compounds in which the affinity for both of these states is equal is known as noncompetitive inhibitors.
It is clear that both uncompetitive and mixed groups of inhibitors belong to a larger group of allosteric inhibitors, but are there any allosteric competitive inhibitors? Surprisingly, yes! Competing for an active site with substrate does not require attaching to it. Actually, some competitive inhibitors control changes of protein states through allosteric site [5]. The only requirement for such kind of inhibitor to be competitive in this case is that the substrate is not bound to an enzyme at the same time the inhibitor is bound to its allosteric site.
Allosteric vs orthosteric inhibition
Since we already know basics of allosteric (indirect) regulation, let’s compare it to the orthosteric (directly to active site) inhibition to consider its advantages.
To begin with, allosteric inhibition has more variability in how and when it affects an enzyme. Interacting with a different from active site area of a protein surface can have not only fully inhibit enzymatic activity (as in case of orthosteric inhibition), but also modulate it [6]. Moreover, many allosteric modulators can interact with an enzyme after connecting of substrate, while orthosteric inhibitors need a free active site to bond with.
Secondly, active site of an enzyme is highly conservative. This leads to orthosteric drugs connecting to homologous proteins with a similar active site instead of their target [7]. Non-target activity not only reduces effectiveness of a drug, but also can result in serious side effects. In contrary, allosteric drugs bind to less conservative surface regions, which allows them to have higher specificity.
Finally, most known orthosteric inhibitors mechanically block an active site of an enzyme, occupying a substrate’s place of interaction. If concentration of substrate is somehow increased, effectiveness of drugs attaching to the same site can be lowered. Allosteric inhibitors, on the other hand, can avoid such difficulties, and their efficiency is not affected by high concentration of substrate [8].
To sum up, allosteric inhibitors have an unusual way of interaction with proteins. They are, in many cases, a safer and more efficient option as drugs than other inhibitors. Currently, their area of use is huge – and it is expanding! ChemDiv’s Allosteric Kinases Inhibitors Library contains 26,000 compounds, which are a promising alternative to current orthosteric inhibitors limited by all the problems discussed above.
References:
- Liu J, Nussinov R. “Allostery: An Overview of Its History, Concepts, Methods, and Applications”. PLoS Comput Biol. 2016 Jun 2;12(6):e1004966.
- Changeux, J.-P. “The Feedback Control Mechanism of Biosynthetic L-Threonine Deaminase by L-Isoleucine”. Cold Spring Harbor Symposia on Quantitative Biology. 1961;26(0), 313–318.
- Wenthur CJ, Gentry PR, Mathews TP, Lindsley CW. “Drugs for allosteric sites on receptors”. Annu Rev Pharmacol Toxicol. 2014;54:165-84.
- Nelson, D. L., & Cox, M. M. “Lehninger principles of biochemistry” (7th ed.). 2017.W.H. Freeman.
- Alphey MS, Pirrie L, Torrie LS, Boulkeroua WA, Gardiner M, Sarkar A, Maringer M, Oehlmann W, Brenk R, Scherman MS, McNeil M, Rejzek M, Field RA, Singh M, Gray D, Westwood NJ, Naismith JH. “Allosteric competitive inhibitors of the glucose-1-phosphate thymidylyltransferase (RmlA) from Pseudomonas aeruginosa”. ACS Chem Biol. 2013 Feb 15;8(2):387-96.
- Nussinov R, Tsai CJ. “The different ways through which specificity works in orthosteric and allosteric drugs”. Curr Pharm Des. 2012;18(9):1311-6.
- Grover AK. “Use of allosteric targets in the discovery of safer drugs”. Med Princ Pract. 2013;22(5):418-26.
- Verma S, Pandey AK. “Factual insights of the allosteric inhibition mechanism of SARS-CoV-2 main protease by quercetin: an in silico analysis”. 3 Biotech. 2021 Feb;11(2):67.


