ADME
ADME: trade fundamentals
PILLars
Four principal components controlling what happens to the pill – or any other drugs – after they are taken. i.e. pharmacokinetics, not to be confused with pharmacodynamics, which describes the other lane of interaction: what drug does to the patient. They are absorption, distribution, metabolism and excretion (ADME for short). In this short piece we will focus on the basics, as an introduction for professionals coming from other fields.
Physicochemical Influences
Before talking about ADME itself, we have to briefly discuss some physical and chemical concepts – the ones that heavily impact ADME. To that end, we will go through lipophilicity, hydrogen bonds, polar surface area and the famous and infamous rule of five.
Lipophilicity
It is common knowledge that oil and water do not mix, so it is expected that the molecule’s lipophilicity, measured by a partition coefficient P (simply the ratio of equilibrium concentrations in 1-octanol and water), impacts everything from solubility to clearance. For the sake of convenience, log P is usually written instead.
If partition is high (the molecule is more soluble in 1-octanol), receptor and enzyme binding is increased, while decreasing its water solubility. Additionally, such molecules tend to better binding to CYP450 enzymes, thus increasing the chance of drug-drug interactions. It also affects plasma protein binding.
Hydrogen Bonds
Hydrogen bonds between a hydrogen and an electronegative atom like oxygen or nitrogen may either form within a single molecule or between different ones. Especially important are hydrogen bonds between the drug and water molecules, because they have to be broken before forming new ones, so substances with too many hydrogen bonds have trouble working their way from the GI tract into the bloodstream.
Polar Surface Area
The size of the molecule, of course, is also a significant factor. It is, unfortunately, hard to measure, so molecular weight, electron density and polar surface area (PSA) are used instead. PSA is the total surface of surface polar atoms. PSA is the most impactful drug absorption metric: if the molecule it to be carried through cells, the upper limit on the size is 120 square angstroms; and for central nervous system drugs the limit is even lower, just 70 square angstroms.
Rule of five
Lipinski and others determined that drugs are (the majority of them, at least) small and lipophilic, so one should be wary of heavy (more than 500 daltons) drug candidates with a large log P (more than 5) and too many hydrogen bond acceptors (more than ten) or donors (five). Due to its straightforward nature, this rule is widely employed during drug discovery.
However, there are drugs beyond these rules and their share on the market is fairly high for modern drugs, so this is not a guarantee, more of a really good guideline.
On a slightly different note, 95% of drugs do not make it through the brain-blood barrier. Usually, higher capacity for hydrogen bonding is a problem. Lipinski also has a rule for drugs intended for central nervous system use: even lower weight than before (400 dalton), log P less than 5, even fewer hydrogen bond donors (less than four) and acceptors (less than eight).
A stands for Absorption
There are three phases to the oral medicine’s travel through the patient: absorption phase (with absorption being the dominant process), post-absorption (elimination is prevalent), elimination (no absorption at this stage).
Conventional parameters for this journey (figure 1) are: Cmax, the maximum concentration or peak concentration; tmax, the time to reach Cmax; Cmin, the minimum concentration observed and AUC – area under the curve, which corresponds to bioavailability. Another, more abstract than physical parameter, is volume of distribution Vd – dose divided by concentration. Dose is just the total amount of drug, while concentration is specifically about plasma. Clearance refers to the blood volume cleaned per time unit. Lastly, there is half-life – 0.693 times volume of distribution divided by clearance.
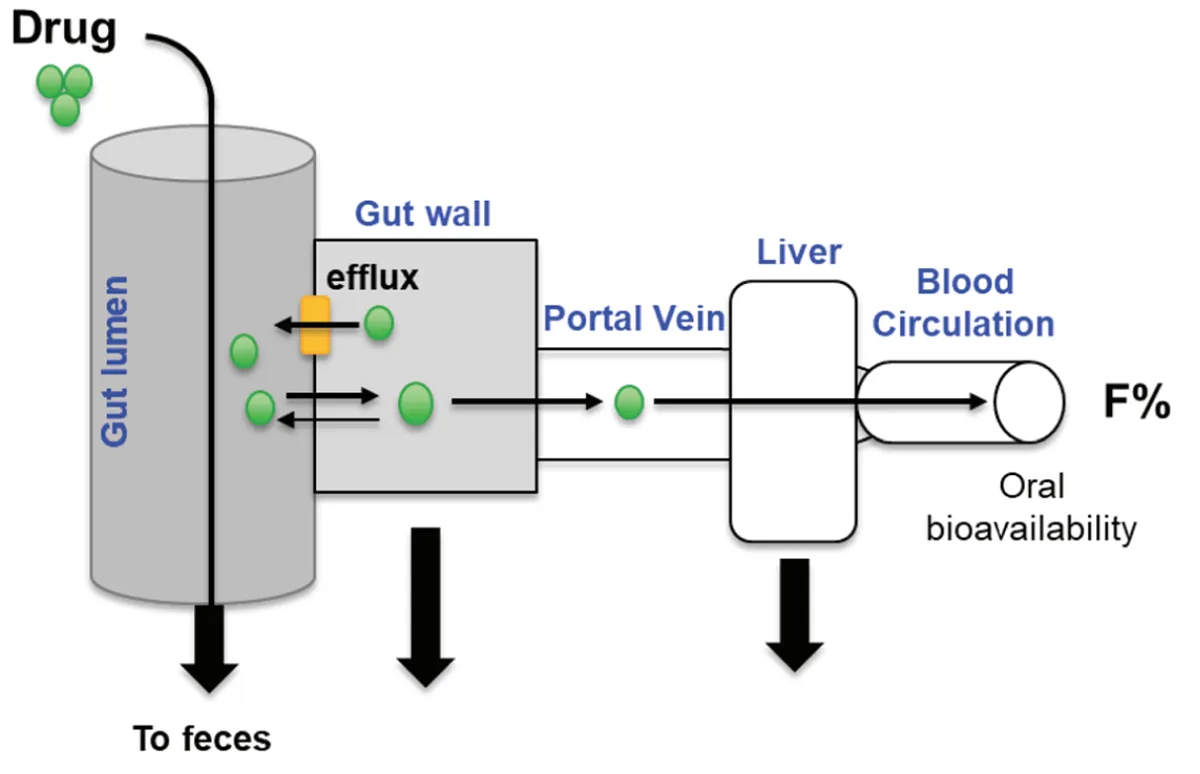
The second figure shows absorption in a single scheme, highlighting the fact that drugs end up going through the liver before becoming part of the general bloodstream, so one should always keep the ways liver interacts with the drug in mind.
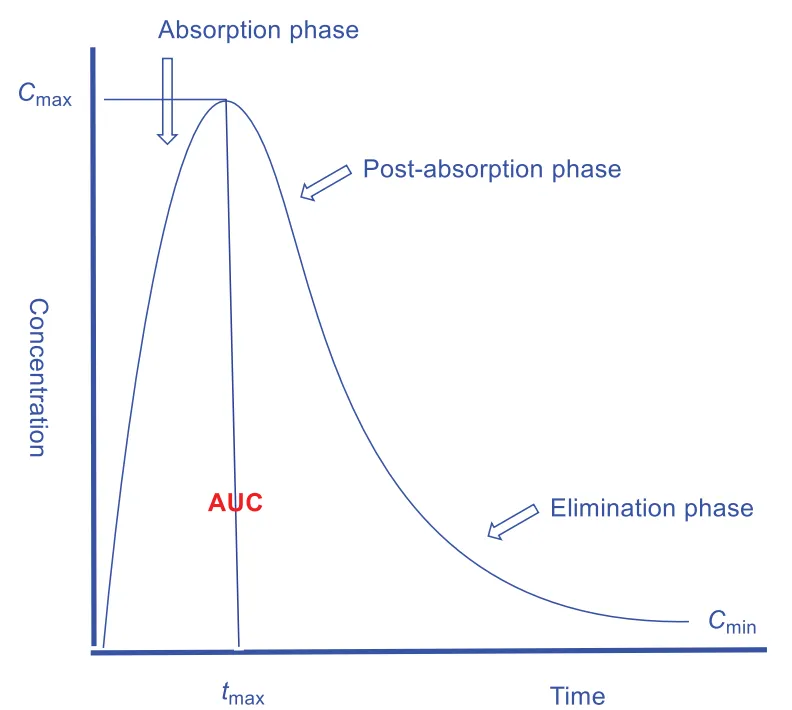
Figure 1. A standard concentration-time graph for oral drugs.
D is for Distribution
After being absorbed, drug is distributed through the bloodstream to the tissues. Distribution denotes the process of the medicine reversibly leaving the blood and going to the tissue. For a sense of scale, plasma only accounts for 5% body mass, while fat, intestinal fluid, intracellular fluid and transcellular fluid account for 20%, 16%, 35%, 2% correspondingly.
Principally, the entirety of body water has three categories: intravascular, interstitial, intracellular. Drugs will be distributed into these three differently depending on their nature and may gather in organs – most notably, in liver or kidneys. And lipophilic drugs will gather in fat tissue.
The entirety of the drug itself is compartmentalized over numerous membranes, tissue, blood and physiological volumes.So if the volume of distribution is extremely high, for example, fifteen thousand liters (assuming the weight of seventy kilograms), one should understand that as the most of the substance concentrated in the tissue.
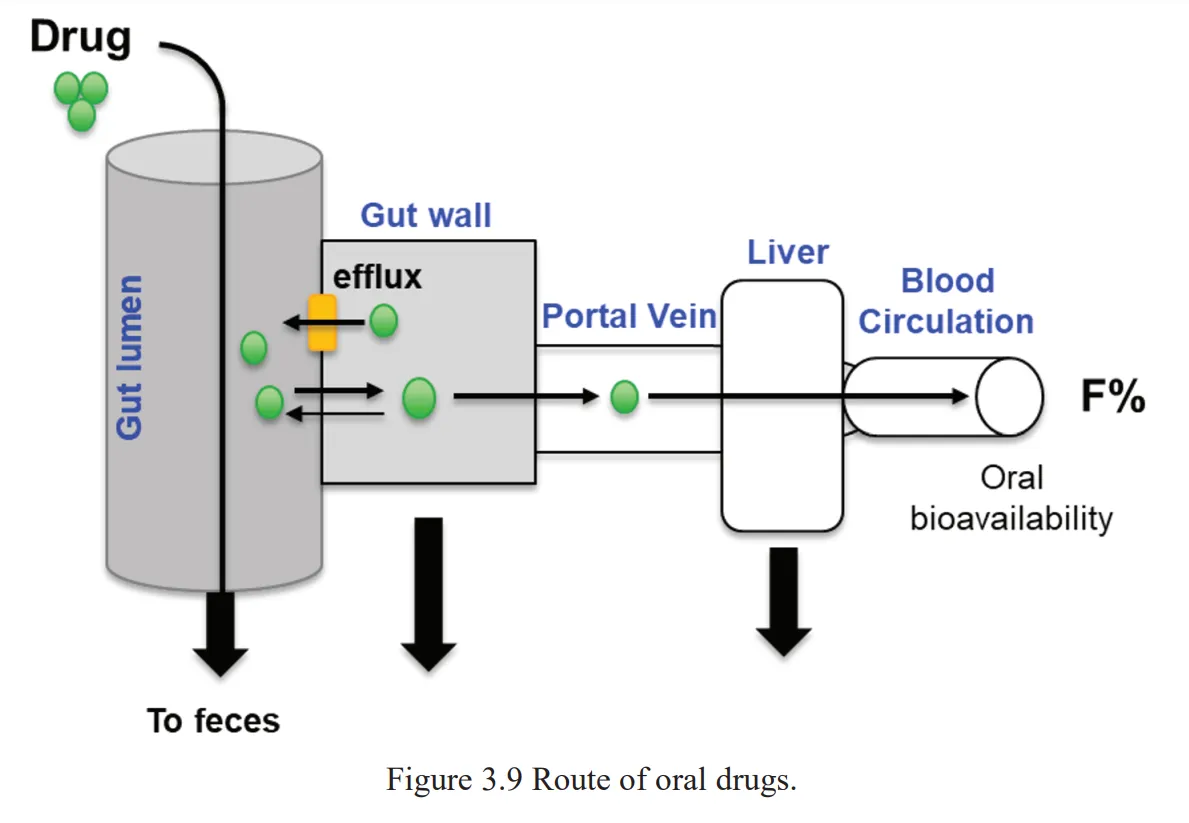
Figure 2. The absorption and early distribution of a drug, with metabolism happening in the liver
Metabolism in the middle
Unless metabolized, lipophilic and some hydrophilic drugs would end up just accumulating in the body. Generally, lipophilic substances are turned into hydrophilic metabolites, which are easier to get rid of. The indisputably main player here is liver, but other sites (kidneys, plasma, intestines, lungs) also play a role. While most compounds are transformed into inactive metabolites, some are converted into active ones. In fact, some medicines actually turn into their active form in the liver, with the molecule consumed beforehand being a prodrug.
On the molecular level, CYP450 enzyme class is largely responsible for drug metabolism. Out of these, 3A4 does around half of the work. Other enzymes from the same family are 2D6, 1A2, 2C9, 2C19 – together with 3A4 they handle 90% of drugs.
There are two phases of drug metabolism, with the first one transforming the functional groups, with the second one consisting of adding a very hydrophilic component to the original molecule or the resulting product of the first phase (figure 3).

Figure 3. Two phases of metabolism
Phase one metabolism can generally be described as a process of oxidation: from hydroxylation to oxygenation at sulfur atoms. These reactions are largely done by liver monooxygenases.
Second phase metabolism consists of conjugation with a substrate to form an easily eliminatable substance.
Ends with Excretion
After dealing with all of that, it is time to get rid of the drug – by elimination of excretion. Most of the time, they go through the renal route and end up in urine. When it comes to kinetics, there are nonlinear (first-order) and linear (zero-order). Most drugs follow nonlinear kinetics, so the constant fraction is taken out each hour, meaning that the elimination rate is concentration-dependent. For linear kinetics, the same amount of drug is eliminated each hour. Any drug at high concentration may behave that way.
Renal route has three parts to it: glomerular filtration, tubular reabsorption and tubular secretion.
For the first one, filtration only goes one way, and, unless the drug is connected to plasma proteins, gets filtered. Most, however, come back with the help of tubular renal cells, provided they are not lipid-soluble or too ionized. After that, tubular secretion occurs, with pumps helping out, finally getting rid of the drug.
Not everything is eliminated through the renal route: liver puts some of the drug into bile, which is released into the GI tract.
Closing remarks
Hopefully, this short article on ADME was sufficient for your purposes. If not, we recommend using our source for this piece – Medicinal Chemistry for Practitioners by Jie Jack Li, pictures are also from there – it goes into much more detail on the matter.


