Drug discovery
Drug discovery: from hit identification to FDA approval
This short review focuses on the overall drug discovery journey (figure 1) – from idea to an FDA request. These journeys take up to fifteen years and cost up to and more than a billion dollars, so there is a lot to cover. In the interest of brevity, a lot will be mentioned, rather than discussed – a more comprehensive, albeit still concise way of learning about drug discovery is to go through the sources for this piece.
.webp)
Figure 1. Drug development timeline, from [1].
We will use an imaginary drug – magic pill, or ‘mpil’ for short – and go through all the stages: finding the target and the corresponding hit, screening – virtual ang high-throughput, hit-to-lead and lead optimisation – these comprise the early development stages; in vitro and in vivo assays, ADME and IND-enabling studies – the preclinical stage, which culminates with an IND application; three clinical phases followed by an FDA review and continuous post-market monitoring.
Development stages
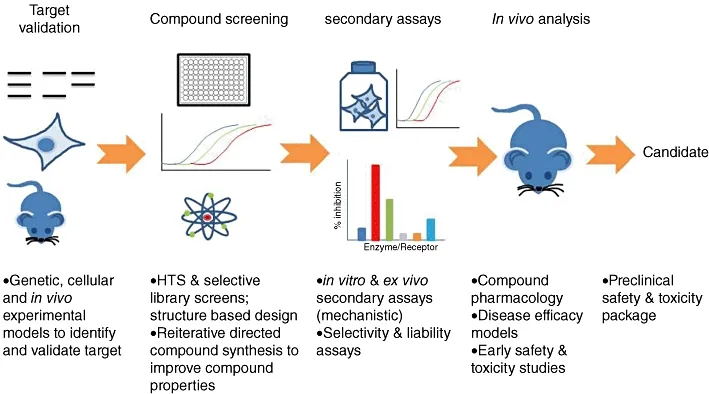
Early stages include everything leading up to the creation of a possible active pharmaceutical ingredient – a drug candidate. Some of the stages presented here may be repeated upon hitting a wall in the later stages – to lower the toxicity, for example.
Target acquired
While what counts as a target is reasonably broad (an enzyme, a gene, an RNA molecule), the goal here is to find something that our future mpil – which is not even an idea at this stage – will be able to have an effect.
Regulatory parts of cell cascades are a common target – we might target kinases; receptors are how cells communicate – we check those for possible targets and so on. The understanding of the disease – both the cause and the symptoms – as well as the fundamental knowledge of how the body functions, reign supreme.
For definiteness, after studying the diseased – by yet another disease, ‘yad’ for short – tissue and cells, we found out that there is a yad-associated kinase that we wish to have an inhibitor for.
.webp)
Figure 2. Further target identification strategies, from [1].
Hitting the mark
After finding the target, the focus turns to finding a molecule – usually relatively small – that is capable of inhibiting the yad-kinase. To that end, common strategies include looking through existing drugs to find something with a similar target to alter (importantly, alter in ways that allow for a separate patent), rational design and CADD – computer-assisted drug design. Most of the time, some combination of strategies is utilized.
In our imaginary case, we used a virtual library of similar compounds to dock with the kinase and stop it from working. After that, we, while looking at decisions made by other researchers, subtly changed some of its outer groups and altered the molecule to increase the rigidity of some of the key parts.
Seeing through the smoke screen
Here, hit series (groups of hits, decided on at the previous stage) are further studied and efficacy examined in available in vivo models. Typically, this translates into SAR investigations and using data given by NMR and crystallography to fuel the molecular modeling techniques employed. After that, an assay cascade (figure 3) with a high-throughput assay at the helm (to weed out the inactive compounds) slowly makes its way towards more advanced stages with different assays, which, as stated above, will, unfortunately, not be discussed here.
.webp)
Figure 3. Possible assay cascade, from[1]. Some text removed.
Lead zeppelin taking off
Even though our lead is identified, we need not stop there. Lead optimization takes place. The methods used here often are not completely unlike ones used at the stage of creating the hits themselves, with full utilization of CADD and assay testing feedback loop. After settling down with a sufficiently promising lead, we proceed to the preclinical stages. If something goes wrong there, we can always come back for additional optimization.
Preclinical stages
An additional overview of early-medium drug discovery stages is given in figure 4.
.webp)
Figure 4. Drug discovery methodology, with the focus on the preclinical stage, from [1].
ADME
Here, the absorption, distribution, metabolism and excretion are studied – essentially, what does the body do to the drug. Additionally, some toxicity studies are conducted. We already did a more in-depth piece on ADME, so we will only give the short version. Absorption, for oral drugs, happens in the GI tract – this includes our mpil. The ability to get through the wall and survive (or deliberately use) early liver metabolism are explored. After the bloodstream is entered, the drug is distributed across the body. Some drugs tend to concentrate in the blood, some in organs, some in tissue – seeing as the drug often needs to go through the cell membrane, some lipophilicity is expected. After doing its work, mpil is metabolized by the liver and then excreted through the renal route, with both of these processes being the target of separate studies.
IND
An IND – or investigational new drug application – is the way through which the exemption from rules barring the crossing state lines is granted by the FDA. At this stage the case for human testing is made, as the next stages are clinical trials.
Clinical stages
First phase
The main goal of the first phase is to check how the volunteers are affected – around fifty are enough. It is at this stage that we hammer out the dosage – our previous estimates were, at best, based on pigs. We also zero in on the safety – what the side effects are, at what dosage they occur, how severe they are.
As it turns out, mpil has only a few mild side effects for healthy volunteers. This is no surprise, only after several months, most drugs pass this stage.
Second phase
The second phase entails testing the drug on a couple hundred people with the disease. This phase is still not large enough to serve as a good test for the usefulness of the drug, giving us additional safety information instead. While, from this description, one might be tempted to call this a repetition of the first phase, after months (up to two years) go by, only around a third pass.
Luckily, while mpil showed significantly more side effects at this stage, it is still within reasonable boundaries, so can continue onto the last phase.
Third phase
Finally, the drug reaches the step during which it can demonstrate its usefulness. This is the largest stage, with up to thousands of participants, all with the disease the drug aims to treat. Additionally, here the less common or long-term side effects are unearthed. After up to four years, only 25-30% pass.
FDA review and post-market
FDA review
Now that we have enough evidence that mpil is both safe and effective – two crucial qualities for any drug – we can file an FDA application, in which we will include all information about mpil to date. Since we are the ones writing the story, we get the good ending – the mpil was approved, now is on its way to patients.
Post-market monitoring
One simply cannot know everything about the drug’s safety just after approval stage, so the monitoring continues months – or even years – to slowly have the entire perspective.
Closing remarks
The drug discovery and approval take a lot of time and resources, but going through everything from early development stages, preclinical and clinical trials, FDA review and post-market monitoring gives humanity a new tool to combat disease and make life better.
Being fully committed to these goals, ChemDiv provides stellar drug discovery services.
Literature
[1] Principles of early drug discovery; Hughes et al., British Journal of Pharmacology, 2011
[2] https://www.fda.gov, last checked 2022


